Peroxidase from Coleus Forskohlii: Purification and Biochemical Characterization
Abstract
In this study, a peroxidase from new source was purified using ion exchange and gel filtration techniques. The recovery for peroxidase activity was 19% with 11-fold purification and specific activity of 749 unit/mg protein. Purified peroxidase demonstrated a molecular mass of 39 kDa using gel filtration and was confirmed as a single band on SDS-PAGE. The purified peroxidase revealed a broad optimum pH activity at 6.0-6.5 and 50°C temperature. The kinetic parameters for purified peroxidase toward H2O2 and guaiacol as substrates were found to be Km = 3.355, 5.395 mM, Kcat = 99.52, 79.56 s-1 and Vmax =1.531, 1.242 µmole ml-1 min-1, respectively. The catalytic efficiency (kcat/Km) of the purified peroxidase was 14.75 and 29.66 s−1 mM−1 for guaiacol and H2O2, respectively. Peroxidase activity was observed to be enhanced by Cu2+, Co2+, Ni2+ and inhibited in the presence of Sn2+, Al3+, Hg2+, NaN3, EDTA and urea. Characterization showed that peroxidase purified from C. forskohlii has the ability to be used for food industrial applications.
Author Contributions
Academic Editor: Saktimayee Roy, Northwestern University, USA
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Yaaser Q. Almulaiky, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interest exists.
Citation:
Introduction
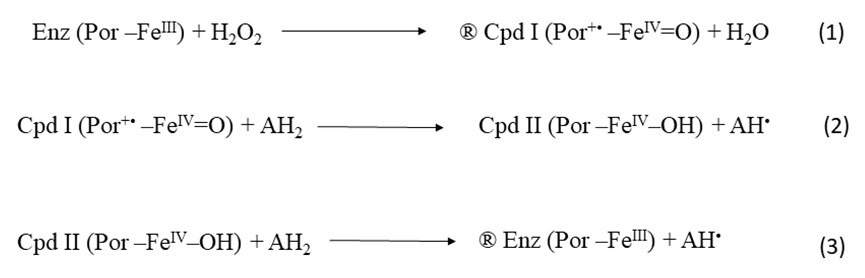
Medicinal plants have been used for a long time since the advent of human civilisatin 1. It is an old practice to use these plants for their medicinal benefits. Their roots in the world's oldest cultures remain well-preserved for many decades 2. C. forskohlii is a persistent medicinal plant of the Lamiaceae family and has been distributed throughout the world, including Saudi Arabia, China, India, Africa and Pakistan 3, 4. An extract of this plant was used in Ayurvedic medicine for centuries to treat different health problems 5. Forskolin, the main diterpene component of this plant, is used as an antioxidant compound for various disorders 6. The antioxidants, including enzymatic antioxidants, can help the body defend itself from various kinds of reactive oxygen damage caused by a variety of conditions. 4. Peroxidase (E.C.1.11.1.7) is belong to the oxidoreductases family, normally found in plants 7, 8, and responsible for the browning process 9. Peroxidase can facilitate plant darkening, although the existence of electron-acceptor compounds such as lipid peroxides, hydrogen peroxide, and superoxide radicals are limits it 10, 11. The catalytic reaction of peroxidases takes place in three different steps. The initial phase includes oxidation of peroxidase to create an unstable intermediate compound called (Cpd I) (1). In the second phase, Cpd I is reduced to Cpd II and to a free radical by an appropriate electron donor (2). Then the second substrate further reduces Cpd II to recover the resting state of the enzyme and another radical one (3) 12.
Specific physiological roles of peroxidases in plants include suberisation, lignification, wound healing, catabolism of auxins, porphyrin metabolism, hydrogen peroxide scavenging, organogenesis and senescence 13. In the presence of hydrogen peroxide, they catalyze the oxidation of different phenolic and nonphenolic compounds 14. Because of their diverse substrate specificity, availability, and high sensitivity, Plant peroxidase has been commonly used in several applications, including environmental protection, such as phenolic wastewater bioremediation, bio pulping, and clinical diagnosis, etc 14. In this study, the enzymatic properties of peroxidase have been studied from a new source. peroxidase from C. forskohlii was purified using ion exchange and gel filtration chromatography. The purity of the enzyme was checked using SDS-PAGE. Also, the physio biochemical properties of the purified enzyme were achieved.
Material and Methods
Chemicals and Plant Materials
Guaiacol, Sephacryl S-200, CM-Sepharose were acquired from Sigma-Aldrich. All other chemicals used were of analytical grade. Coleus forskohlii is readily available because of its ubiquitous growth in the wild. The sample was obtained from Khulais, Saudi Arabia, in Sep. 2019, situated at 39°21'50"E110"22'58°21.
C. forskohlii Peroxidase Purification
Preparation of the Plant Extract
Fifteen grams of Coleus forskohlii peel stem had been ground in a 0.02 M Tris–HCl buffer, pH 7.2. Filtered and centrifuged this crude extract for 15 min at 12,000 rpm and discarded the pellet.
Ion Exchange and Gel Filtration Chromatography
Briefly, CM-Sepharose filled the column, equilibrated with 0.02 M Tris–HCl buffer, pH 7.2. The homogenous enzyme was then loaded onto the column and eluted at a flow rate of 30 mL/h in the same buffer with a gradient of 0.0–0.3 M NaCl. Four peaks were pooled with protein fractions exhibiting peroxidase activity. The fraction with the highest activity were applied on a Sephacryl S-200 column equilibrated with 20 mM Tris–HCl buffer (pH 7.2). peroxidase was again eluted at a flow rate of 30 mL/h and quantities of 3 ml have been collected.
Protein Estimation
Bradford method 15 used to quantify the protein using bovine serum albumin as standard.
Determination of Molecular Weight
According to Laemmli method 16, the pooled enzyme from gel filtration chromatography was added to SDS-PAGE to check the purity of the enzyme and assess its molecular weight.
Enzyme Assay
The peroxidase activity was determined using H2O2 and guaiacol as substrates 17. The reaction mixture contained 20 μL enzyme sample, 40 mM guaiacol, 8 mM H2O2, and 50 mM phosphate buffer (pH 7.0).
Physicochemical Characterization of the Enzyme
Effect of pH
In the range of pH 4.0–9.0, the peroxidase activity was measured using 50 mM of acetate buffer (pH 4.0–6.0) and Tris/HCl buffer, (pH 6.5–9.0). The stability at the different pH levels (pH 5.0-8.5) was established by calculating the residual activity in buffers with the same pH after incubating the enzyme for 24 h at 4 °C.
Effect of Temperature and Thermal Stability
The temperature effect on purified peroxidase activity was performed at temperatures between 30 and 80 °C. To obtain thermal stability, the enzyme was pre-incubated at the working temperature for 30 min before the addition of substrates and starting the reaction.
Kinetic Parameters
Km, Kcat and Vmax values of peroxidase were determined for hydrogen peroxide and guaiacol substrates. The kinetic parameter values were calculated from Line Weaver-Burk graphs.
Effect of Metal Ions and Inhibitors
The effect of different metals and inhibitors on enzyme activity has also been investigated. Salt additives of CuSO4, CoSO4, NiCl2, AlCl3, SnCl2 and Hg(NO3)2 (2.0–10 mM) have been used in this study. Peroxidase was additionally incubated with several compounds, such as sodium azide (NaN3), EDTA (2.0-10 mM) and Urea (0.2-1 M). The enzyme was pre-incubated with metals and/or inhibitors and buffer for 30 min before adding the substrates. Relative activity, against the control, was estimated. Control of peroxidase activity (without addition of metals and/or inhibitors) was considered to be 100% activity.
Results and Discussion
Purification of C. forskohlii Peroxidase
Prior to use in any industrial process, biochemical characterization of an enzyme is necessary 18. In this study, C. forskohlii peroxidase purification was optimized and its biochemical properties were determined. As can be seen in Figure 1, four major protein peaks (A280) and four C. forskohlii peroxidase peaks have been obtained using CM-sepharose column chromatography. The fraction (POD3) with highest peroxidase activity were collected and concentrated. The purity of C. forskohlii peroxidase (POD3) was 3.5-fold with a cumulative recovery of 24 % (Table 1). Finally, to obtain a homogeneous peroxidase, the enzyme was applied to Sephacryl S-200 column (Figure 2A). The C. forskohlii peroxidase (POD3A) was purified at a yield of 19% and 11-fold purification with specific activity of 742 unit/mg protein. The purified peroxidase provided a single protein band of 12% SDS-PAGE (Figure 2B), confirming its uniformity. Using a Sephacryl S-200 column, the molecular weight of purified peroxidase was estimated to be 39 kDa. Moreover, SDS-PAGE research was carried out to determine and check molecular weight and purified peroxidase uniformity. These findings are in agreement with all those peroxides produced from other plant sources, for example, broccoli (48 kDa) 19, Commiphora gileadensis (40) 8, pearl millet grains (31 kDa) 20. The results obtained from the effect of pH on the C. forskohlii peroxidase showed that the enzyme has a broad optimum pH from 6.0 to 6.5 (Figure 3a). Peroxidases from most plants are particularly active in pHs 4.5–7, but some peroxidases display a lower pH maximum activity (pH 3.8) 21. To assess the effect of pH stability on C. forskohlii peroxidase, the purified enzyme was incubated at various pH ranging from 5.0 to 8.5 for 24 h at 4 °C (Figure 3b). As seen in this figure, C. forskohlii peroxidase activity at pH ranging from 5.5 to 7.5 was relatively higher, which is compatible with the optimal pH study. The findings are in line with an earlier report where it was found that Triticum aestivum peroxidase was stable for two days at different pH (5.0-7.0) 22.
Table 1. Purification scheme of C. forskohlii peroxidase| steps | T. units | T. Protein mg | S.A | Fold purification | Recovery 100% |
|---|---|---|---|---|---|
| Unit/mg protein | |||||
| Crude extract | 725 | 11 | 66 | 1 | 100 |
| Chromatography CM-sepharose0.0 M NaCl (POD1) | 0.8 | 1.5 | 102 | 0.06 | 6.12 |
| 0.1M NaCl (POD 2) | 9.18 | 0.15 | 61.2 | 0.9 | 1.2 |
| 0.2M NaCl (POD 3) | 174 | 0.75 | 232 | 3.5 | 24 |
| 0.3M NaCl (POD 4) | 20 | 0.1 | 200 | 3 | 2.8 |
| Gel filtration on sephacryl S-200 | |||||
| POD 3A | 141 | 0.19 | 742 | 11 | 19 |
Figure 1.A typical elution profile for the chromatography of peroxidase using a CM-Sepharose column.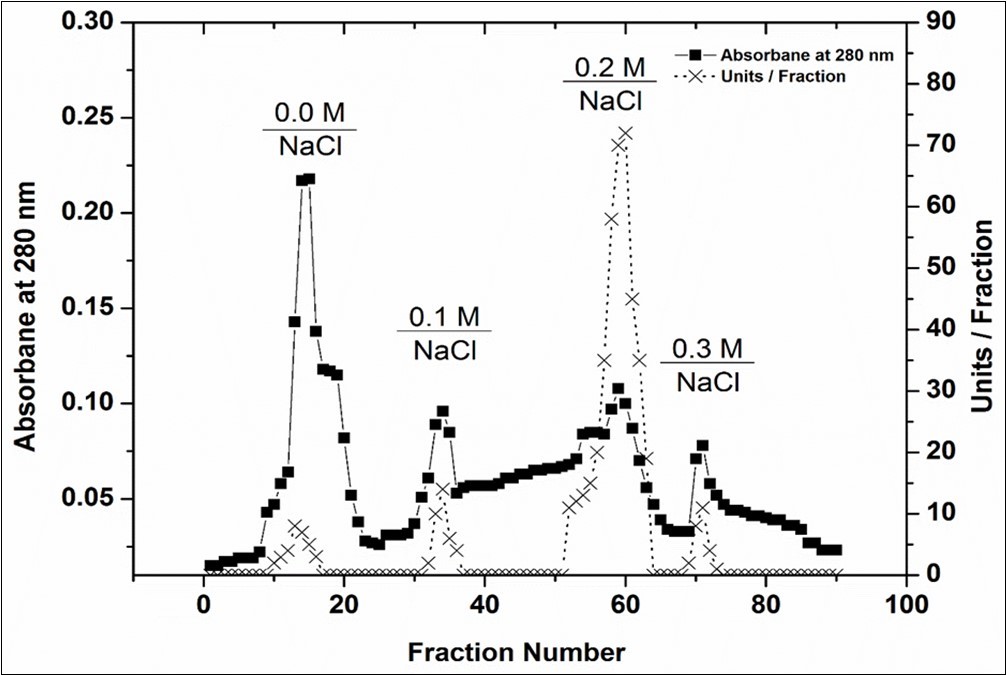
Figure 2.Gel filtration of POD3 CM-Sepharose fractions using a Sephacryl S-200 column (A), SDS-PAGE for homogeneity and molecular weight determination of peroxidase (B).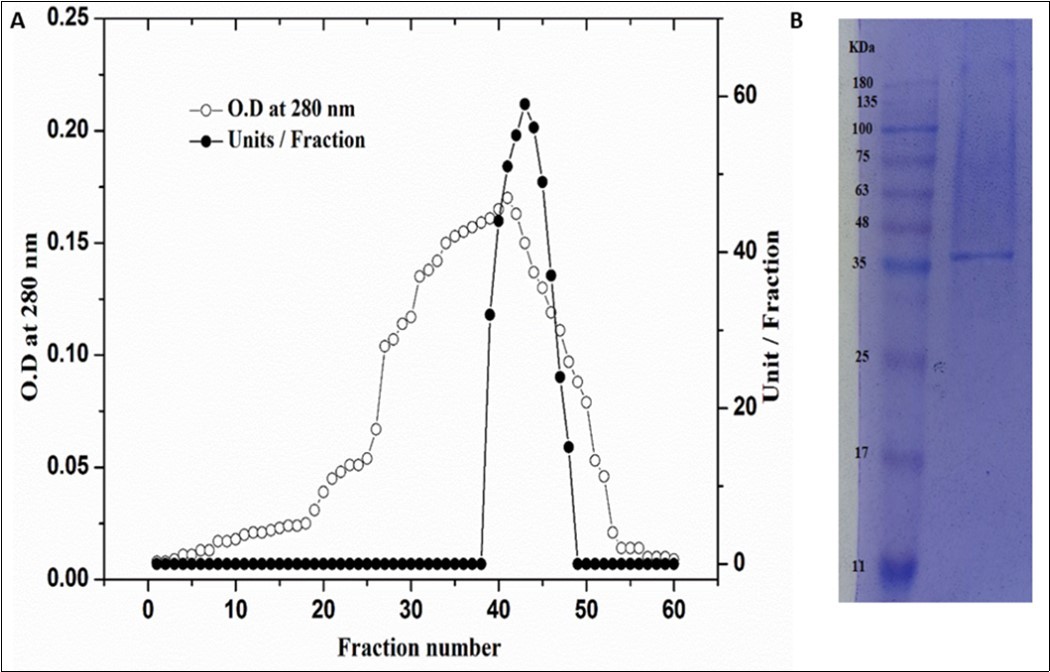
Figure 3.Optimum pH (A), pH stability (B)
The temperature profile revealed the maximum C. forskohlii peroxidase activity at 50 °C temperature and steadily dropped above 50°C (Figure 4a). The purified enzyme retained 63% and 40% of its initial activity at 60 and 70 °C, respectively. Similar findings have been reported for Arabian balsam peroxidase 8, Spinacia oleracea peroxidase 23. The thermostability of the purified enzyme at various temperatures was shown in Figure 4b. The enzyme was robust to 50 °C. At temperatures above 50 °C, the stability of the enzyme gradually decreased. A broad diversity in thermal stability for peroxidase activity has been noted from diverse sources, including date palm leaves (75 °C), 24 horseradish cv. Balady (40°C), 25, chewing stick miswak (40 °C) 26, and Arabian balsam (55 °C) 8.
Figure 4.effect of temperature (A), Temperature Stability (B)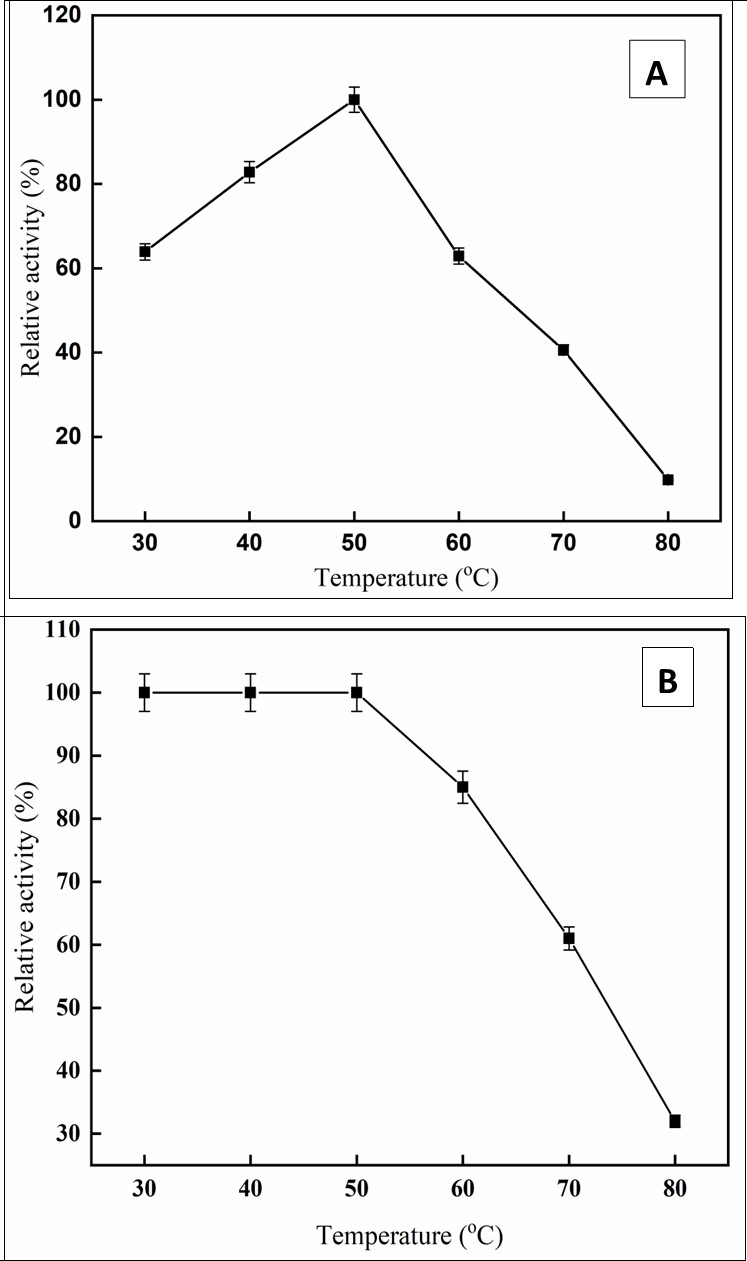
The kinetic constants for guaiacol and Hydrogen peroxide oxidation by C. Forskohlii peroxidase has been calculated and the apparent Km, Kcat and Vmax values are shown in Table 2 (Figure 5 a,b). The C. forskohlii peroxidase provided Km values of 3,355 and 5,395 mM for substrates H2O2 (Figure 5a) and Guaiacol (Figure 5b). The Km values appear to be less than those reported for Triticum aestivum peroxidase (7.3 and 6.6 mM for H2O2 and guaiacol) 27, peroxidase from Arabian balsam (4.81 and 46.5 mM for H2O2 and guaiacol) 8. The low Km values show a high evident affinity to H2O2 and guaiacol in the enzyme relative to previously reported peroxidases. The Vmax values of C. forskohlii peroxidase was 1.531 and 1.242 µmole/ml for H2O2 and guaiacol, respectively. On the other hand, the purified enzyme has Kcat values of 99.52 and 79.56 s-1 for H2O2 and guaiacol, respectively. The catalytic efficiency (kcat/Km) of the purified enzyme using guaiacol and H2O2 was found to be 14.75 and 29.66 s−1 mM−1, respectively (Table 2).
Figure 5.Lineweaver-Burk plots relating purified peroxidase reaction velocity to H2O2 (A) and guaiacol (B) concentrations.
| Kinetic parameter | C. forskohlii peroxidase | |
| H2O2 | Guaiacol | |
| Km (mM) | 3.355 | 5.395 |
| Vmax (µmole ml-1 min-1) | 1.531 | 1.242 |
| kcat (s−1) | 99.52 | 79.56 |
| kcat/Km (s−1 mM−1) | 29.66 | 14.75 |
The effects of metal ions (Cu2+, Co2+ and Ni2+), at a final concentration ranging from 2 to 10 mM, on C. forskohlii peroxidase activity are summarized in Table 3. Such metals have an activation effect on the C. Forskohlia peroxidase activity. Cu2+ and Ni2+ were reported to stimulate peroxidase activity in radish seedlings and Horseradish, respectively 28, 29.
Table 3. Effect of metal salts on C. forskohlii peroxidase| Relative activity % | |||||
| Metal Salts | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM |
| CuSO4 | 102.8 | 106.5 | 114.5 | 123.9 | 118.9 |
| CoSO4 | 104.9 | 109.8 | 111.9 | 111.8 | 112.1 |
| NiCl2 | 102.5 | 103.7 | 109.2 | 124.6 | 129.2 |
The inhibitory effects of sodium azide, three metal ions and organic compounds of EDTA (2-10 mM), and urea (0.2-1 M) on C. forskohlii peroxidase activity are outlined in Table 4. When Sn2+ affectively inhibited purified peroxidase activity, metal ions Al3+ and Hg2+ showed moderate inhibition effects against peroxidase. Each of these metal ions had half maximum inhibition (IC50) concentrations of 1.38, 3.83, and 5.49 mM, respectively. Furthermore, the inhibitor constant (Ki Guaiacol) of the specified metal was determined in accordance with the graph of Lineweaver–Burk, by 0.072, 0.41 and 0.79 mM and (Ki H2O2) by 0.179, 1.01 or 0.196 mM, respectively. Sodium azide and EDTA have moderate inhibitory effect on the purified peroxidase. The half maximum inhibition concentrations (IC50) were calculated as 10.49 and 9.15 mM, respectively. while the inhibitor constant (Ki) were found to be 1.543, 1.187 mM for guaiacol and 3.798, 2.952 mM for H2O2, respectively. Inhibition of peroxidase by Sn2+, Al3+, Hg2+ ions, NaN3, and EDTA were also reported in several studies 27, 30, 31, 32. The direct interaction between a peptide backbone and urea molecule caused protein unfolding. Basically, high urea concentrations caused protein unfolding followed by decreased enzyme activity 33. As shown in Table 4, exposure of purified peroxidase with urea at 0.2-1 M was determined. The half inhibition concentration (IC50) was 0.808 mM, while the inhibitor constant (Ki) were 0.094 mM for Guaiacol and 0.233 mM for H2O2.
Table 4. Inhibition properties of some inhibitors on C. forskohlii peroxidase activity.| Ki (mM) | IC50 (mM) | |||
| Concentration | Guaiacol | H2O2 | ||
| AlCl3 | 2-10 mM | 0.41 | 1.01 | 3.83 |
| SnCl2 | 0.072 | 0.179 | 1.38 | |
| Hg(NO3)2 | 0.79 | 0.196 | 5.49 | |
| NaN3 | 1.543 | 3.798 | 10.49 | |
| EDTA | 1.187 | 2.952 | 9.15 | |
| Urea | 0.2-1 M | 0.094 | 0.233 | 0.808 |
Conclusion
In this study, peroxidase was purified from a new source, C. forskohlii, using ion exchange chromatography (CM-Sepharose), and gel filtration chromatography (Sephacryl S-200). Appropriate conditions for the peroxidase activity have been analyzed and calculated. Peroxidase has been shown to be relatively active under acidic conditions. Km, Kcat, Ki, Vmax and IC50 have been estimated for both substrates. The influences of different metal ions on peroxidase have also been determined. The enzyme performed optimal activity and stability in a temperature at 50 °C. The C. forskohlii peroxidase can potentially be used for bread leavening and bakery, in addition, high-temperature enzyme stability would be useful in these applications.
References
- 1.Gülçin İ, Oktay M, Kireçci E, Öİ Küfrevioğlu. (2003) . , Screening of Antioxidant and Antimicrobial Activities of Anise (Pimpinella anisum L.) Seed Extracts. Food Chem 83, 371-382.
- 2.Oktay M, Gülçin İ, Öİ Küfrevioğlu. (2003) Determination of in Vitro Antioxidant Activity of Fennel (Foeniculum vulgare) Seed Extracts. Lebensm Wissen Technol. 36, 263-271.
- 3.Shukla P K, Misra A, Kumar M, Jaichand S K, Akhtar J et al. (2018) Simultaneous quantification of forskolin and iso-forskolin in Coleus forskohlii (Wild.) Briq. and identification of elite chemotype, collected from eastern ghats (India). , Pharmacogn. Mag 13, 881-885.
- 4.Almulaiky Y Q, Kuerban A, Aqlan F, Alzahrani S A, Baeshen M N et al. (2017) . In vitro Antiglycation, Antioxidant Properties of Coleus forskohlii ''Balady'' Leaves and Stem and their Antioxidant Enzyme Activities. Annu Res Rev Biol 1-11.
- 5.Ding X, Staudinger J L. (2005) Induction of drug metabolism by forskolin, the role of the pregnane X receptor and the protein kinase a signal transduction pathway. , J. Pharmacol. Exp. Ther 312, 849-856.
- 6.Zhang X, Li F, Guo L, Hei H, Tian L et al. (2015) Forskolin regulates Ltype calcium channel through interaction between actinin 4 and β3 subunit in osteoblasts. , PLoS One 10, 0124274.
- 7.Köksal Z, Kalın R, GulçIn İ, Özdemir H. (2018) Inhibitory Effects of Selected Pesticides on Peroxidases Purified by Affinity Chromatography. , Int. J. Food Properties 21(1), 385-394.
- 8.Almulaiky Y Q, Al-Harbi S A. (2019) A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. , Int. J. Biol. Macromol 133, 767-774.
- 9.Cao X M, Zhang Y, Wang Y T, Liao X J. (2011) Effects of High Hydrostatic Pressure on Enzymes, Phenolic Compounds, Anthocyanins, Polymeric Color and Color of Strawberry Pulps. , J. Sci. Food Agric 91, 877-885.
- 10.Chisari M, Barbagallo R N, Spagna G. (2007) Characterization of Polyphenol Oxidase and Peroxidase and Influence on. , Browning of Cold Stored Strawberry Fruit. J. Agric. Food Chem 55, 3469-3476.
- 11.Gülçin İ, Yıldırım A. (2005) Purification and Characterization of Peroxidase from Brassica oleracea Var. , Acephala. Asian J. Chem 17(4), 2175-2183.
- 12.Kharatmol P P, Pandit A B. (2012) Extraction, partial purification and characterization of acidic peroxidase from cabbage leaves. , J. Biochem Technol 4, 531-540.
- 13.Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. (2001) A large family of Class III plant peroxidases. , Plant Cell Physiology 42, 462-468.
- 15.Bradford M M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. , Anal. Biochem 72, 248-254.
- 16.Laemmli U K. (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. , Nature 227, 680-685.
- 17.Yuan Z Y, Jiang T J. (2003) Horseradish peroxidase, in: JR Whitaker, A Voragen, DWS Wong (Eds.), Handbook of food enzymology. , New York 403-411.
- 18.Kumar S, Satyanarayana T. (2003) Purification and kinetics of a raw starch hydrolyzing, thermostable, and neutral glucoamylase of the thermophilic mold Thermomucor indicae-seudaticae. , Biotechnol. Prog 19, 936-944.
- 19.Goyal P, Chugh L K. (2013) Partial purification and characterization of peroxidase from pearl millet (Pennisetum glaucum [L.] r. br.) grains. , Journal of Food Biochem 38(2), 150-158.
- 20.Deepa S S, Arumughan C. (2002) Purification and characterization of soluble peroxidase from oil palm (Elaeis guineensis jacq) leaf. , Phytochemistry 61(5), 503-511.
- 21.Morisco F, Vitaglione P, Amoruso D, Russo B, Fogliano V et al. (2008) Foods and liver health. , Mol. Asp. Med 29, 144-150.
- 22.Altın S, Tohma H, Gülçin İ, Köksal E. (2017) Purification, characterization, and inhibition sensitivity of peroxidase from wheat (Triticum aestivum ssp. vulgare). , Int J Food Prop 20(9), 1949-1959.
- 23.Köksal E. (2011) Peroxidase from leaves of spinach (Spinacia oleracea): Partial purification and some biochemical properties. , Int J Pharmaco 7(1), 135-139.
- 24.Al-Senaidy A M, Ismael M A. (2011) Purification and characterization of membrane-bound peroxidase from date palm leaves (Phoenix dactylifera L.). , Saudi J. Biol. Sci 18(3), 293-298.
- 25.Mohamed S A, Abulnaja K O, Ads A S, Khan J A, Kumosani T A. (2011) Characterisation of an anionic peroxidase from horseradish cv. , Balady. Food Chem 128(3), 725-730.
- 26.Mohamed S A, Al-Malki A L, Khan J A, Sulaiman M I, Kumosani T A. (2012) Properties of peroxidase from chewing stick miswak. , Afr J Pharm Pharmacol 6(9), 660-670.
- 27.Altay A, Koktepe T, Durmaz L, Topal F, Gülçin İ et al. (2018) Purification and selected biochemical properties of peroxidase from cress (Lepidium sativum sub sp. , sativum). Int. J. Food Prop 21(1), 2610-2621.
- 28.Chen E L, Chen Y A, Chen L M, Liu Z H. (2002) Effect of copper on peroxidase activity and lignin content in Raphanus sativus. , Plant Physiology and Biochemistry: PPB / Societe Francaise De Physiologie Vegetale 40, 439-444.
- 29.Nazari K, Mahmoudi A, Khodafarin R, Moosavi-Movahedi A A, Mohebi A. (2005) Stabilizing and suicide-peroxide protecting effect of Ni 2+ on horseradish peroxidase. , J Iran Chem 2(3), 232-237.
- 30.Yuzugullu Karakus Y, Acemi A, Işık S, Duman Y. (2018) Purification of peroxidase from Amsonia orientalis by three-phase partitioning and its biochemical characterization. Sep Sci Technol. 53(5), 756-766.
- 31.Alshawafi W M, Aldhahri M, Almulaiky Y Q, Salah N, Moselhy S S et al. (2018) . Immobilization of horseradish peroxidase on PMMA nanofibers incorporated with nanodiamond. Artif Cells Nanomed Biotechnol 46(sup3), S973-S981 .
Cited by (17)
This article has been cited by 17 scholarly works according to:
Citing Articles:
Separation and Purification Technology (2025) Crossref
Separation and Purification Technology (2025) OpenAlex
Plant and Soil (2024) Crossref
GSC Biological and Pharmaceutical Sciences (2024) OpenAlex
Pakistan Journal of Biological Sciences (2024) Crossref
Pakistan Journal of Biological Sciences (2024) OpenAlex
Plant and Soil (2023) OpenAlex
Electronic Journal of Biotechnology (2023) Crossref
Electronic Journal of Biotechnology (2023) OpenAlex
Biocatalysis and Biotransformation (2022) Crossref
Biocatalysis and Agricultural Biotechnology (2022) Crossref
Biocatalysis and Agricultural Biotechnology (2022) OpenAlex
Catalysis Letters (2022) Crossref
Catalysis Letters (2022) OpenAlex
Biocatalysis and Biotransformation (2021) OpenAlex
Journal of Food Biochemistry (2021) Crossref
Journal of Food Biochemistry (2020) OpenAlex