Abstract
Introduction
The objective of this study was to compare the availability and prices of locally produced and imported medicines, in particular after one year from medicines importation restriction and to answer the key questions, did local manufacturers able to coverage national needs of medicines and what is the patient prices for locally produced compared to imported medicines in different sectors and regions of Sudan.
Methodology
The WHO/HAI methodology survey tool was adapted to measure the availability and price of locally produced and imported medicines. Patient price and availability were collected from capital cities of 6 states as per WHO/HAI methodology. Data were collected and analyzed for 50 medicines from the 104 medicines restricted to local manufacturer. Availability was based on whether the medicine was in stock on the day of data collection at the surveyed facility. Prices were expressed as median price ratio (MPR).
Results
Availability of locally manufactured medicines (LMM) was much better than imported medicines (IM), in the public, (47.2% vs. 14%, respectively) and private (63.9% vs. 23.5%, respectively) sectors. Based on median price ratio (MPR), public sector patient prices for locally manufactured medicines were lowered priced and had a median MPR of 2.4 (n=42) than imported medicines which had a median MPR of 4.99 (n=20). In private sector patient prices for locally manufactured medicines were also lowered priced and had a median MPR of 2.76 (n=45) than imported medicines which had a median MPR of 5.53 (n=27). Thus; patients were paying about 52% less for locally produced than for imported medicines in both sectors
Conclusion
The survey showed low availability of the basket of medicines surveyed in the public and private sectors for imported medicines (I.M), while not achieving WHO’s target of 80 % for locally manufactured medicines (LMM). In developing countries a lot of barriers are well known to business and industrial need to be resolved in order to maintain availability and self-reliance in drug production as a mean of increasing access to medicines.
Author Contributions
Academic Editor: Lucio Mango, University Master, University of International Studies (UNINT) – Rome, Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Salah I. Khder, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Drugs are often the most important cost of health care expenditure in management process of diseases. Patients that have access to adequate and effective drugs at the time of need are most likely to be happy with treatment they receive. The availability of affordable and effective drugs is, therefore, one the most visible indicators of the quality of health services. Despite significant progress in increasing access to essential medicines in low-and middle income countries during the past decades, many o the health services still lack adequate supplies of basic medicines. Drug shortage and quality problems continue to undermine the performance of health systems through the developing world. 1 Policy makers in developing world particularly Africa are increasingly exploring and promoting local industrial production of pharmaceutical and medical supplies and looking for improvement on coverage and affordability of quality assured medicines that meet local health needs. Meanwhile, low-income population in Sub-Saharan Africa (SSA) continue to suffer severely inadequate and exclusionary health care undermined by poor access to medicines and supplies.2 Median availability of essential medicines 2007 was only 60% overall, and 56% in the public sector of low and lower middle income countries (LMICs) and have changed little in SSA countries, despite major funding efforts for HIV and TB medication. 3, 4 Sudan’s epidemiological profile is typical of Sub-Saharan African countries; malnutrition and communicable diseases dominate the health scene with high vulnerability to outbreaks. 5 Analysis of health system financing in Sudan indicates that 65% of funding is from private sources, almost all of which is out-of-pocket expenditure. 6 Medicines supply maintained by both public and private sectors. In public sector procurement is carried out by the National Medical Supply Fund (NMSF) through national and international tenders and awarded items supplied mainly to federal hospitals and public health facilities. In private sector medicines provided through either local manufacturer which cover only 30% of Sudan market or through direct importation from international companies by local agents and contribute to supply the remaining 70% of medicines need in private sector in Sudan. There is a relatively significant domestic pharmaceutical industry, with 24 licensed pharmaceutical manufacturers operating in the market, all of finished dosage forms, but not active pharmaceutical ingredients. However, the basic capabilities of the local drug makers will make it difficult to achieve self-sufficiency and the supply of more sophisticated medicines will remain mandatory by imports. This current lack of capacity is highlighted by the fact that only 17% of registered pharmaceuticals are produced locally. In Sudan, the law requires marketing authorization (registration) for all pharmaceutical products before marketing from the National Medicines and Poisons Board (NMPB). Manufacturers are licensed; overseas manufacturers are inspected every five years against WHO/GMP guidelines whereas local companies are inspected yearly. Importers, wholesalers/distributors, and pharmacies are also licensed. Prices and mark-ups were regulated throughout the supply chain. Sudan does not apply value added tax (VAT) on medicines. However, import duties of 8% are applied to all imported medicines. Import duties are also applied to API’s. Mark-up profit is (15%) for both importer and local manufacturer. 7, 8 In 2016, the number of pharmaceutical products registered in Sudan was 4641. The majority of medicines imported from foreign pharmaceutical companies mainly from Jordan (18%), India (13%), Pakistan (9%), Egypt (8%), European countries (7%), China (7%), Japan (7%), Saudi Arabia (6%), and UAE (4%). 9
Increasingly government is supporting local medicine production, expecting that it will result in increased availability and lower prices, as well as industrial and economic benefits. 10 A presidential decree issued in July 2017 ordered that not to allow any locally produced medicines from being imported from outside Sudan. Accordingly in January 2018; National Medicines and Poisons Board (NMPB); the regulatory authorization in Sudan issued a list of medicines which produced by local industries and supposed to be 'self sufficient' of these medicines, this list is composed of 104 human medicines of different pharmacological group restricted only to local manufacturers and blocked from importation. 11, 12
The objective of this study was to compare the availability and prices of locally produced and imported medicines, in particular after one year from medicines importation restriction and to answer the key questions, did local manufacturers able to coverage national needs of medicines and what is the patient prices for locally produced compared to imported medicines in different sectors and regions of Sudan?. The WHO/HAI methodology survey tool was adopted with some variations in terms of identification of surveyed medicines by substituting generic and branded medicines with locally produced and imported medicines, to measure their availability and price in order to answer the study questions. For better understanding availability and prices of selected medicines were collected from different regions in Sudan. The data obtained were analyzed and compared and presented as results from public and private sectors and discussed in details in the forthcoming sections. Such studies provide valuable advocacy messages for policymakers, pharmaceutical industries, regulators, prescribers and patients if well shared and delivered in a timely manner.
Methods
Study Design
Sampling
Patient price and availability were collected from capital cities of 6 states as per WHO/HAI methodology. The survey areas were Khartoum capital, Omdurman and Khartoum North cities representing central and peripheral Khartoum State, Wad-madni city representing Gezera State, Al-Obid city representing North Kordfan State, Alomglad city representing South Darfur State and Port-Sudan city representing Red Sea State. 30 public sector outlets were sampled (hospital pharmacies and health facilities) and 30 private retail pharmacies.
Medicines
Data were collected and analyzed for 50 medicines from the 104 medicines restricted to local manufacturer (Table 1). The medicines were selected nationally, with strength- and dosage form- specific, and which made by at least one local manufacturer. In each outlet, for each medicine data were collected on all products in stock with the same active ingredient (s), strength and dosage form. Of 50 medicines surveyed, 9 were global medicines, 9 regional medicines and 32 were supplementary medicines.
Table 1. Medicines Surveyed| Analgesics & Anti-inflammatory drugs | Blood supplements |
|---|---|
| Paracetamol 24 mg / ml suspension | Pyridoxine Hcl 40 mg cap/tab |
| Paracetamol + caffeine 500 + 65 mg cap/tab | Cardiovascular drugs |
| Paracetamol + Chlorzoxazone 300 + 250 mg cap/tab | Amolodipine 5 mg cap/tab |
| Paracetamol 500 mg tab | Atenolol 50 mg cap/tab |
| Ibuprofen 400 mg tab | Atorvastatin 20 mg cap/tab |
| Diclofenac 50 mg tab | Atorvastatin + Amolodipine 20 + 10 mg cap/tab |
| Anti-diabetic | Bisprolol fumerate 2.5 mg cap/tab |
| Glibenclamide 5 mg tab | Candesartan Cilextil 16 mg cap/tab |
| Metformin 500 mg tab | Candesartan Cilextil + Hydrochlorothizide 16 + 12.5 mg tab |
| Anti-infectives | Furosemide 40 mg cap/tab |
| Albendazole 200 mg tab | Lisinopril 10 mg cap/tab |
| Amoxicillin 500 mg tab | Valsartan/Hydrochlothiazide |
| Amoxicillin suspension 50 mg/ml millilitre | Acetyl Salicylic Acid 100 mg cap/tab |
| Ampicillin + cloxacillin 500 mg cap/tab | CNS drugs |
| Azithromycin 250 mg cap | Diazepam 5 mg cap/tab |
| Azithromycin Dry Powder 200 mg /5ml millilitre | Resperidone 1 mg cap/tab |
| Cefixime 100 mg milliliter | Quetiapine Fumarate 300 mg cap / tab |
| Cefixime caps 400 mg cap/tab | Gastro-intestinal drugs |
| Cephalexin 500 mg cap/tab | Alu.Hydroxide + Mg: Trisilicate 250+ 120 mg cap/tab |
| Ciprofloxacin 500 mg cap/tab | Esomeprazole 20 mg cap/tab |
| Co-trimoxazole suspension 8+40 mg /ml milliliter | Hyoscin 10 mg cap/tab |
| Co-trimoxazole Tabs 480 mg cap/tab | Loperamide 2 mg cap/tab |
| Doxacyclin 100 mg cap/tab | Omeprazole 20 mg cap/tab |
| Metronidazole Suspension 40 mg / ml milliliter | Ranitidine 150 mg cap/tab |
| Metronidazole 250 mg tab | Erectile Dysfunction |
| Praziquantel 600 mg tab | Sildenafil Citrate 25 mg cap/tab |
| Quinine Sulphate 300 mg tab | Respiratory system drugs |
| Antihistamines | Cough Syrup (Any formula) 2.5 mg + 125 mg milliliter |
| Chlorophenarmine maleate 4 mg cap/tab | Salbutamol 4 mg cap/tab |
Data Collection and Entry
Data were collected by 6 pharmacy students of final year at National University-Sudan as a part of their graduation project. Data were checked at the end of each area survey by the student's supervisor for completeness and possible errors. Prices were identified from packs or companies invoices. Data entered into the automated Excel workbook generated by WHO/HAI, but modified for the sake of the study objectives.
Data Analysis
For the purposes of study analysis, local production was identified as the lowest price generic and defined as products that were manufactured and packaged/labeled in Sudan. On the other hand imported drugs were identified as branded medicines which include both originator and generic medicines imported totally from outside Sudan. Availability was based on whether the medicine was in stock on the day of data collection at the surveyed facility. All medicines were included in the availability analysis and calculated as percentage in which medicines was found on the date of data collection. Prices were expressed as median price ratio (MPR). An MPR is the ratio of the price in local currency (Sudanese Pound SDG) divided by the international reference prices (IRP) converted to international currency using the exchange rate of costing adopted by NMPB during the period of study (1 USD = 30 SDGs). The MPR is thus an expression of how much greater or less the price in the country than IRP.An MPR of 1 or less is taken as efficient procurement in the public sector, while below 3 is considered acceptable for the private sector. 13 The Workbook calculated the MPR for each medicine type in each sector only if the medicine was available in at least four facilities.In this survey, use of IRPs serve as a benchmark for price comparisons between locally produced and imported medicines. The IRP were taken from the 2007 Management Sciences for Health International Drug Price Indicator Guide. These are the medians of recent bulk procurement or tender prices offered by profit and nonprofit suppliers to developing countries for multisource products. Univariate analysis was performed to determine the price variation (median, interqurtile range and range) for median MPR for patient and regions surveyed for both locally manufactured medicines (LMM) and imported medicines (IM).
Results
Availability and Prices in the Public Sector
In the public outlets surveyed, locally manufactured medicines (LMM) was more predominant than imported medicines (IM), but in general the mean percentage availability of all surveyed medicines in the public sector was low at 14.0% for (IM) and 47.2% for (LMM). There was a big variation in mean availability among the regions surveyed. The lowest for IMs availability was seen in Port-Sudan city (Eastern State) at 2.0% and for LMMs was seen in Wadmadni city (Gezera State) at 32.0%. Availability for IMs was highest in the Peripheral of Khartoum city (Capital State) at 29.6% and for LMMs was highest in S. Kordfan (Western state) at 62.4%. Figure 1 shows the mean availability in each survey region. For individual medicines (Table 2) shows the percentage availability of individual medicines grouped into bands. For imported drugs (IM); 10 of IMs were not found in any of 30 public outlets surveyed (0.0%), while most of IMs 19 were available in the range of (1.0%-10.0%). However; none of them available as 50.0% or above (highest available medicines were Diclofenac & Omeprazole 46.7%). For LMMs only one medicine not available in any of the surveyed public outlets (Atorvastatin + Amlodipine) while majority of them available as ≥40%. Amolodipine tabs was the highest available as locally manufactured medicine (83.3%).
Figure 1.Cross regional mean availability (%) for IMs and LMMs public sector
| Availability band % | Imported Medicines | Locally Manufactured Medicines |
| 0.0% | Amoxicillin Suspension, Ampicillin + Cloxacillin, Atenolol, Cephalexin, Cotrimoxazole tab, Hyoscine, Ibuprofen, Praziquantel, Quitapine Fumerate, Quinine Sulphate, | Atorvastatin + Amlodipine, |
| 1.0%-10.0% | Acetyl Salicylic Acid, Albendazole, Alu. hydroxide + Mg, Trisilicate, Atorvastatin + Amlodipine, Chlorophinamine, Co-trimoxazole suspension, Diazepam, Loperamide, Metronidazole Suspension, Metronidazole, Paracetamol Suspension, Paracetamol + Caffeine, Paracetamol tab, Pyridoxine Hcl, Resperidone, Salbutamol , Sildenafil, Tetracycline ,Valsartan Hydrochlorthiazide. | Albendazole, Diazepam, Quitapine Fumerate, Resperidone, Sildenafil Citrate, Valsartan Hydrochloride |
| 11.0%-20.0% | Atorvastatin, BisprololFumerate, Esomeprazole, Furosemide, Glibenclamide, Paracetamol + Chlorzoxazone . | Alu. Hydroxide + Mg. Trisilicate, |
| 21.0%-30.0% | Amoxicillin, Azithromycin, Azithromycin Dry Powder, Candesartan Cilextil + Hydrochlorothiazide, Cefixime Caps, Ciprofloxacin, Ranitidine. | Candesartan Cilextil + Hydrochlorothiazide, |
| 31.0%-40.0% | Amlodipine, Candesartan Cilextil, Cefixime Dry Powder, Cough Syrup (Any formula), Lisinopril, Metformin. | Azithromycin Dry Powder, Co-trimoxazole Suspension, Co-trimoxazole tabs, Esomeprazole, Lopermide, Paracetamol + Caffiene, Paracetamol + Chlorzoxazone, Quinine Sulfate, Salbutamol. |
| 41.0%-50.0% | Diclofenac, Omeprazole, | Ampicillin + Cloxacillin, Candesartan Cilextil, Furosemide, Glibenclamide, Metronidazole, Praziquantel, Ranitidine, Tetracycline. |
| 51.0%-60.0% | - | Amoxicillin, Atenolol, Azithromycin, Cefixime caps, Cephalexin, Diclofenac, Ibuprofen, Lisinopril, Omeprazole, Paracetamol. |
| 61.0%-70% | - | Chlorphenarmine maleate, Ciprofloxacin, Hyoscin, Metformin, Paracetamol Tabs, |
| 71.0%-80.0% | - | Acetyl Salicylic Acid, Atorvastatin, BisprololFumerate, Cough Syrup (Any formula), Metronidazole Suspension |
| 81.0%-90.0% | - | Amlodipine. |
| 91.0% - 100.0% | - | - |
Public sector patient prices for locally manufactured medicines were lowered priced than imported medicines and had a median MPR of 2.4 (n=42) while imported medicines had a median MPR of 4.99 (n=20). The median brand premium was 2.3 (meaning that, on average, IM were about 2.3 times the price of LMM). Hence patients were paying about 107% times the price of the LMM (on average) more when being dispensed as imported products in public sector.
Twenty (20) matched pairs of medicines were found for comparison between imported medicines and locally manufactured medicines equivalents. The MPRs for IMs and LMM are shown in Figure 2, which shows how much more IMs were priced compared to their LMM equivalents. Of both IM and LMM in public sector, Esomeprazole was found to be the highest median MPR (49.81 and 14.27 respectively). The regional comparison for IM showed that Khartoum Peripheral had the lowest median MPR for (1.77), while they were highest in Port-Sudan city (4.01) Table 4. The 25th and 75th percentiles were 2.84 and 8.53 respectively, indicating a larger variation across the pharmacies compared to LMM 1.52 and 4.02.
Figure 2.Medicine price ratio in public sector for paired IMs and LMMs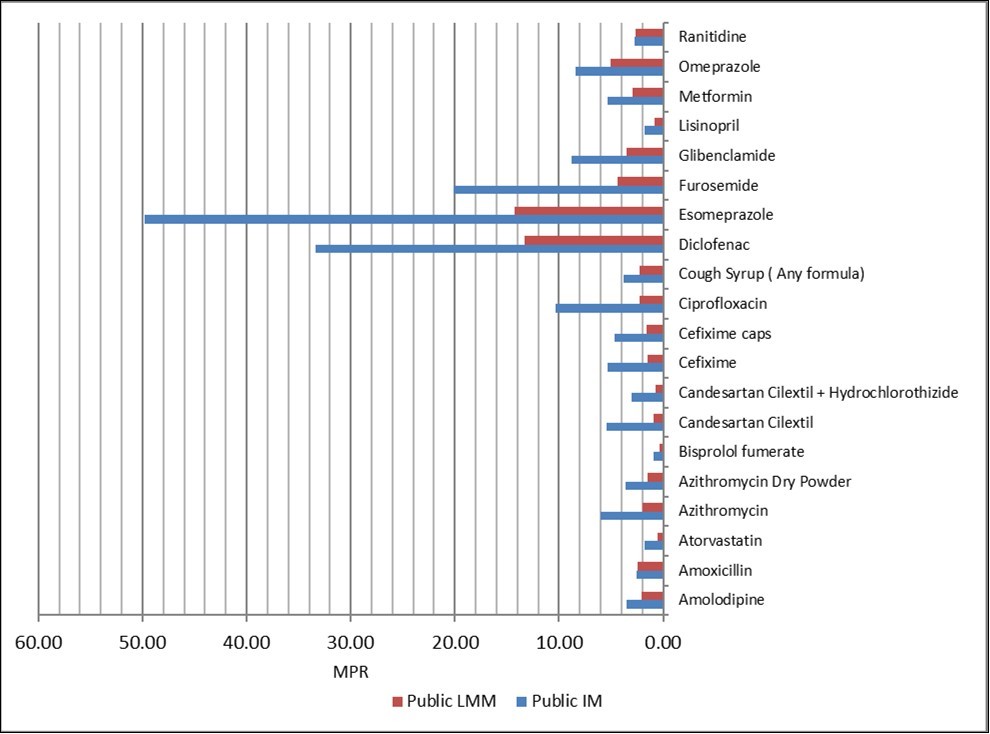
Availability and Prices in the Private Sector
Again local manufactured medicines availability in the private outlets surveyed was higher than in the imported Medicines, with better availability in private sector than public sector. The mean availability of LMM was (63.9%) and for IM was (23.5%). Mean availability varied across the 6 regions surveyed. The lowest availability was also seen in Port-Sudan city (Eastern State) at (4.8%) and for LMMs in Wadmadni city (Gezera State) at (54.7%). Also Availability for IMs was highest in the Peripheral of Khartoum city (Capital State) at 40.0% and for LMMs was highest in S. Kordfan (Western state) at (79.5%). Figure 3 shows the mean availability in each surveyed region.
Figure 3.Cross regional mean availability (%) for IMs and LMMs private sector
For individual medicines (Table 3) shows the percentage availability of individual medicines grouped into bands. For imported medicines (IM); 8 of IMs were not found in any of 30 private outlets surveyed (0.0%), while cluster of IMs (22 items) were available in the range of (1.0%-10.0% & 10-20%), 11 items in each range. Highest available medicines was Amolodipine tabs 81.5%. For LMMs only one medicine lowest available medicine in private outlets surveyed was Quitapine fumerate (3.7%) while majority of them available clustered in the range of (61.0-70.0%);13 items and ( 81.0%-90.0%); 12 items. Atenolol, Bisprolol fumerate, Cefixime caps were the highest available medicines as locally manufactured medicine (92.6% each).
Table 3. Availability% (in bands) of medicines in private outlets surveyed| Availability band % | Imported Medicines | Locally Manufactured Medicines |
|---|---|---|
| 0.00% | Amoxicillin Suspension , Atenolol, Atorvastatin + amlodipine, Chlorphenarmine maleate, Co-trimoxazole tabs, Hyoscin, Resperidone. | |
| 1.0%-10.0% | Ampicillin + Cloxacillin, Cephalexin, Co-trimoxazole Suspension, Ibuprofen, Lopermide, Paracetamol + Caffiene, Paracetamol + chlorzoxazone , Paracetamol Tabs, Praziquantel, QuitapineFumerate, Quinine Sulfate. | Quitapine fumerate. |
| 11.0%-20.0% | Albendazole, Diazepam, Furosemide, Glibenclamide, Metronidazole Suspension, Metronidazole, Ranitidine, Salbutamol, Sildenafil Citrate, Tetracycline, Valsartan/Hydrochlorthiazide. | Atorvastatin + Amlodipine, Resperidone, Valsartan/Hydrochlorthiazide |
| 21.0%-30.0% | Acetyl Salicylic Acid, Alu. Hydroxide + Mg Trisilicate, Amoxicillin, BisprololFumerate, Esomeprazole, Paracetamol, Pyridoxine Hcl. | Diazepam. |
| 31.0%-40.0% | Azithromycin Dry Powder, Cough Syrup (Any formula). | Albendazole, Paracetamol + Chlorzoxazone, |
| Pyridoxine Hcl, Quinine Sulfate, Sildenafil Citrate. | ||
| 41.0%-50.0% | Atorvastatin , Azithromycin, Cefixime Caps, Ciprofloxacin. | Candesartan Cilextil + Hydrochlorothiazide. |
| 51.0%-60.0% | Candesartan Cilextil + Hydrochlorothiazide, Cefixime Dry Powder, Diclofenac | Alu. Hydroxide + Mg. Trisilicate, Azithromycin Dry Powder, Co-trimoxazole Tabs, Diclofenac, |
| Paracetamol + Caffiene, Praziquantil. | ||
| 61.0%-70% | Candestan Cilextil, Lisinopril, Metformin, | Amlodipine, Ampicillin + Cloxacillin, Cephalexin, Chlorophenarmine maleate, Cough Syrup (Any formula), Esomeprazole, Furosemide, Glibenclamide, Lisinopril, Metronidazole, Omeprazole, Salbutamol, Tetracycline. |
| 71.0%-80.0% | Omeprazole . | Azithromycin, Candesartan Cilextil, Hyoscin, Loperamide, Metformin, |
| 81.0%-90.0% | Amlodipine. | Acetyl Salicylic Acid, Amoxicillin, Amoxicillin suspension, Atorvastatin, Cefixime, Ciprofloxacin, Co-trimoxazole suspension, Ibuprofen, Metronidazole Suspension, Paracetamol, Paracetamol Tabs, Ranitidine, |
| 91.0% - 100.0% | - | Atenolol, Bisprololfumerate, Cefixime caps, |
Private sector patient prices for locally manufactured medicines were lowered priced and had a median MPR of 2.76 (n=45) than imported medicines which had a median MPR of 5.53 (n=27). The median brand premium was about 1.95 (meaning that, on average, IM were about two times the price of LMM). Hence patients were paying about 95% times the price of the LMM (on average) more when being dispensed as imported products in private sector.
Twenty seven (27) matched pairs of medicines were found for comparison between imported medicines and locally manufactured medicines equivalents. The MPRs for IMs and LMM are shown in Figure 4, which shows how much more IMs were priced compared to their LMM equivalents. Of both IM and LMM in private sector, Esomeprazole also was found to be the highest median MPR (65.01and 18.15 respectively).
Figure 4.Medicine price ratio in private sector for paired IMs and LMMs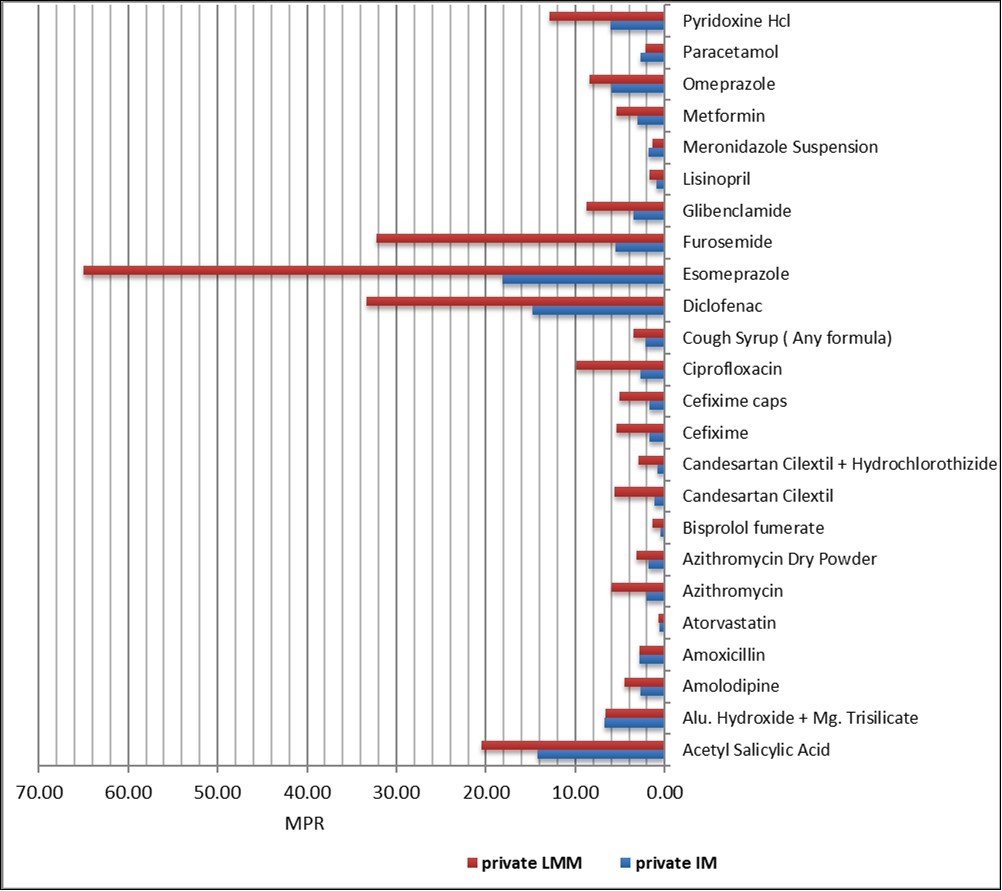
The regional comparison for LMM showed that Khartoum Peripheral had the lowest median MPR for (2.11), while they were highest in the Wadmadni city (3.56). The 25th and 75th percentiles were 1.85 and 5.11 respectively. For IM; the lowest median MPR also in Khartoum Peripheral (3.48); while the highest in Khartoum central (5.34). The 25th and 75th percentiles were 2.89 and 9.3 respectively, indicating a larger variation across the pharmacies compared to LMM.
Across 20 medicines paired analysis patients paying more in private sector by 4.6% than public sector for imported medicines. However, across 42 medicines paired analysis patients paying more for locally imported medicines in private sector by 14.8 % than public sector. This percentage difference between private to public varies among regions for different paired items and it reaches up to 27.8% for imported medicines in peripheral of Khartoum and 41.5% for locally manufactured medicines in Wadmadni city Table 4.
Table 4. Univariate analysis Comparing median MPR for patient & region surveyed for both LMM & IM in pair.| Public | Private | ||||||
| IM | LMM | IM | LMM | % diff private to public IM | % diff private to public LMM | ||
| Patient | Number of paired medicines | 20 | 42 | 27 | 45 | ||
| Prices | Median MPR | 4.9 | 2.4 | 5.35 | 2.76 | 4.6% | 14.8% |
| Median interquartile range (IQR) or (The 25th and 75th percentiles) | 2.94-8.53 | 1.52-4.02 | 2.89-9.3 | 1.85-5.11 | |||
| Min MPR | 0.91 | 0.33 | 0.76 | 0.47 | |||
| Max MPR | 49.81 | 16.67 | 65.01 | 27.57 | |||
| Region | Median MPR Khartoum Centeral | 1.99 | 5.26 | 5.34 | 2.32 | 7.1% | 12.3% |
| Prices | Median MPR Khartoum peripheral | 1.77 | 4.73 | 3.48 | 2.11 | 27.8% | 10.0% |
| Median MPR Wadmadni | 2.73 | N.P* | 8.44 | 3.56 | - | 41.5% | |
| Median MPR Obid | 1.78 | N.P* | 6.28 | 2.68 | - | 30.1% | |
| Median MPR S. Kordfan | 2.22 | 4.89 | 3.98 | 2.61 | 0.0% | 1.4% | |
| Median MPR Port-Sudan | 4.01 | N.P* | N.P* | 3.13 | - | 0.0% | |
Discussion
One of the suggested pathways towards the removal of barriers to quality drugs is the development, or strengthening, of local production systems. Other objectives for industrial policy of local production: the desire to develop a local employment base; the need to increase technology transfer; the wish to become 'self sufficient' in medicines; the need to reduce reliance on imports and manage foreign exchange flow; and the desire to produce medicines for export. 14 The objective of this paper to provide insight into the availability, price and affordability of medicines in Sudan, comparing the availability and price of locally produced and imported medicines in public and private sectors in different regions in the country, through the use of the WHO/HAI medicine prices survey which allowed the measurement of medicine prices and availability in a reliable and standardized way that enables valid systemic comparisons to be made.
The survey showed low availability of the basket of medicines surveyed in the public and private sectors for imported medicines (I.M), while not achieving WHO’s target of 80 % for locally manufactured medicines (LMM). In the public sector outlets, locally produced products were far more commonly stocked than imported products (47.2% compared to 14%). In the private sector, outlets tended to stock more locally produced products (63.9%) compared to imported products (23.5%). In all six survey regions the availability of locally produced products was higher than for imported products in the public and private sectors. Similar results were obtained from Ethiopian survey where locally produced medicines are more predominant than imported medicines, while opposite results were obtained from Tanzanian survey, that imported medicines are more available than locally produced medicines. 15 In developing countries particularly Sub-Saharan countries a lot of barriers are well known to business and industrial concern affecting availability and self-reliance in drug production as a mean of increasing access to medicines; such as a weak financial sector (banking/non banking); diminished availability and flows of foreign currency for investment; inflation; taxes; shortage of technical expertise; non stable supply of electricity, gas and other utilities. Also manufacturing settings activities are limited to compounding and packaging, and processing bulk medicines into dosage forms using imported raw materials; on average 40-50% of their cost of goods sold tied up to raw material costs which in turn need to be paid for in foreign exchange. Indeed, machinery, technology, quality control equipment, advertising and distribution networks must often be purchased for foreign exchange. 14 & 16
The survey results show that patients are paying significantly more to purchase imported medicines than locally manufactured medicines in all sectors surveyed. While noting the WHO target that consumers should pay no more than four times the IRPs, we observed that medicine prices were higher for imported medicines compared to IRPs in both public and private sector (4.99 and 5.53 respectively). Overall patients were paying 107% more in public sector and 95% more in private sector for imported products compared to locally produced products. Some individual imported medicines were being purchased at very high prices than locally produced ones e.g. Esomeprazole 20 mg cap were dispensed as imported medicines at a price that was 3.5 times than locally produced price in both public and private sectors.
Compared to information on medicine prices and availability in general, little is known about the impact of local medicine production on prices and availability in different countries. In many countries, studies found locally produced medicines had lower patient prices compared to imports. Kuanpoth found locally produced ARVs had lower patient prices compared to imported ARVs in Vietnam. 17 Chowdury and Kabir found locally produced over-the-counter essential medicines in Bangladesh had lower patient prices compared to imports. 18 Sweileh et al. found lower patient prices for antibiotics made locally compared to imports. 19 One study, conducted by Shafie and Hassali in Malaysia, found some locally produced generics had higher patient prices compared to imports. 20
The apparent consumer willingness to pay higher prices for imported products, as seen in the public and private sector in Sudan, may reflect a perception that imports are of higher quality. To boost local industries, the government needs to ensure and publicise the equivalent quality of locally produced medicines, also the local pharmaceuticals need to spend more money in promotion and marketing of their products and do not depends only on price difference from imported medicines. Supporting local manufacturers through fiscal and/or non-fiscal incentives must be time-bound, developed and implemented in a transparent way, and should not be implemented suddenly or abruptly otherwise it may unintended had a negative effects on the availability. Balancing local production policies is critically important and information on foreign exchange, exports, imports, job demand and other economic and societal indicators need to be evaluated before and after creating local manufacturing capacity; and before and after changes in industrial and/or pharmaceutical policy with regard to local production of pharmaceuticals.
Finally; In-spite of all obstacles facing local production we have to recognize that many advantages of local production for both public health and economic development could be achieved such as improve affordability of quality medicines from known and frequently inspected facilities. It has also the potential to offer other advantages over imports; for example by shortening related supply chains; it can help reduce stock outs and by expanding and diversifying the supply chain, it allows developing countries to secure access in response to growing demands. There is also some evidence that locally manufactured products are more successful in reaching rural populations than imported ones.
Strategic and logistic limitations in our research may have affected the findings . Thus, three main limitations arose, firstly; we did not include the procurement sector, and identifying prices and availability of imported and locally produced medicines procured by the government, secondly we were not differentiate between prices and availability of imported medicines by product type (originator brands, branded generics) and thirdly; patient affordability have not been measured furthermore, price components in the supply chain.
Conclusion
In both the private and public sectors, considerable price differences were seen between LMM and IM. In general, IM were almost 2 times more expensive than the LMM.The availability of the surveyed medicines was extremely low in all sectors as imported medicines and better as locally manufactured medicines.The impact of policy changes made should be measured by establishing a monitoring system to monitor not only regularly the prices, but also the availability and affordability of medicines
References
- 1.Berber M, Murugi J, Buch E. (2010) Strengthening Pharmaceutical Innovation in Africa. Geneva and Johannesburg: Council on Health Researches for Development and The New Partnership for Africa's Development (NEPAD) Agency of Africa Union.
- 2.Wagner A K, Graves A J, Reiss S K. (2011) Access to Care Medicines, Burden of Health Care Expenditure , and Risk Protection: Results from The World Health Survey. Health Policy. 100, 151-158.
- 4.Nations United. (2015) Taking Stock of the Global Partnership for. Development Millennium Development Goal 8 MDG Gap Task Force Report , New York .
- 5.World Health Organization. (2014) Local Production for Access to Medical Products. Developing a Framework to Improve Public Health. Geneva: WHO, Essential Medicines and Products: brief;.
- 6. (2016) Health Finance Policy Options for Sudan. Khartoum (FMOH), Sudan:Public Health Institute, Fedral Ministry of Health.
- 8.Sudan Medicines Index. Khartoum, National Medicines& Poisons Board (2013) . Available at:http://www.nmpb.gov.sd/DisplaySearch2.php
- 9.General Pharmacy Directorate.(FMOH),Annual Health Statistical Report. Khartoum, Sudan:National Health Information Centre, Federal Ministry of Health(2016).
- 10. (2016) FMOH.National Health Sector Strategic Plan II (2012-16).Khartoum, Sudan. Fedral Ministry of Health.
- 12. (2018) . List of locally Satisfied of Human Medicines from National Pharmaceutical Industries,Phase1 .
- 13.Gelders S, Ewen M, Noguchi N, Laing R. (2006) Price, availability and affordability: An international comparisons of chronic disease medicines. World Health Organization and Health Action International. , Cairo, Available
- 14.W Laig Kaplan, R. (2005) Local production of pharmaceuticals: Industrial policy and access to medicines- an overview of key concepts, issues and opportunities for future research. Health, Nutrition and Population (HNP) Discussion Paper,Washington,DC:World Bank.
- 15.Kaplan W, Gedif T, Justin-Temu M, Vialle-Valentin C, Mirza Z et al. (2017) Prices and availability of locally produced and imported medicines in Ethiopia and Tanzania. Journal of pharmaceutical policy and practice. 10, 1-9.
- 16.Consoli Davide.literature review on local production of medicines and access to health‐care. Project no. 513396. Consoli-Background -Paper.pdf .
- 17.Kuanpoth J. (2007) Patents and access to antiretroviral medicines in Vietnam after World Trade Organization accession. , J World Intell Prop 10, 201-24.
- 18.Chowdury N, Kabir E R. (2009) Per pill price differences across therapeutic categories: a study of the essential drug brands marketed by multinational and local pharmaceutical companies in Bangladesh. , African J Marketing Mgt 1, 220-6.
Cited by (3)
- 1.Nakoma-Ngoma Theresa, Leslie John F., Monjerezi Maurice, Mvumi Brighton M., Chamboko Tafireyi, et al, 2025, Increasing adoption of grain postharvest technology by smallholder farmers: a five-pronged strategy, Frontiers in Sustainable Food Systems, 9(), 10.3389/fsufs.2025.1640274
- 2.Hemmeda Lina, Tiwari Angad, Kolawole Barakat Olajumoke, Ayoobkhan Fathima Shehnaz, Fatima Kainat, et al, 2024, The critical pharmaceutical situation in Sudan 2023: A humanitarian catastrophe of civil war, International Journal for Equity in Health, 23(1), 10.1186/s12939-024-02103-9
- 3.Gutema Girma, Homa Gadissa, 2022, Cropping Up Crisis at the Nexus Between COVID-19 and Antimicrobial Resistance (AMR) in Africa: A Scoping Review and Synthesis of Early Evidence, Cureus, (), 10.7759/cureus.21035
