Unusual cutaneous toxicity after prolonged use of Hydroxyurea in polycythemia vera: a case report
Abstract
Hydroxyurea (HU) is commonly used for the long-term treatment of patients with chronic myeloproliferative neoplasms. The drug is usually well tolerated in the large majority of subjects, although systemic and/or localized toxicities have seldom been reported. Here we report a case of unusual cutaneous toxicity due long-term use of HU in a patient with polycythemia vera.
Author Contributions
Academic Editor: Andreas Petzer, Head Internal Medicine I: Medical Oncology, Hematology and Gastroenterology, Barmherzige Schwestern Hospital Linz.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Elisa Molinari MD, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Hydroxyurea (HU) is a hydroxylated derivative of urea first synthesized in Germany by Dressel and Stein in 1869 and commonly used for the long-term treatment of patients with Philadelphia-chromosome negative chronic myeloproliferative neoplasms (MPNs). Still today HU is considered a first choice agent for the treatment of MPNs disorders, as underlined by the European Leukemia Net Group guidelines1. The drug is commonly well tolerated in the large majority of subjects, although systemic and/or localized toxicities have been reported2. Mucocutaneous toxicity related to hydroxyurea has been well described since many years, although severe toxic effects are unusual. Among the most frequently described mucocutaneous complications of HU treatment diffuse hyperpigmentation, alopecia, melanonychia, onycholysis, dermatomyositis-like lesions and stomatitis with aphthous ulcers are reported3. Serious cutaneous adverse events include skin cancer and ulcers. These latter lesions are however rare, and even rarer are the localization on hands and head. Here we report a case of unusual and late cutaneous toxicity due to HU observed in our Center.
Case presentation
In November 2010 a 86 years old man was firstly seen in our outpatient Clinic because of polyglobulia. The patient was complaining for intense pruritus since a few months. In the medical history the patient was reportedly affected since several years by gouty arthropathy, lower limb arterial disease, chronic obstructive pulmonary disease and arterial hypertension. Physical examination was unremarkable; in particular no splenomegaly or lymphadenomegalies were observed. The hematocrit was 58% and hemoglobin concentration was 19g/dl, with otherwise normal white cells and platelet’s counts; biochemistry was unremarkable, with the exception for serum lactate dehydrogenase, which was significantly increased. The pulmonary function tests showed only a mild obstructive pattern of anomaly; arterial blood gas values indicated moderate hypoxia but serum endogenous erythropoietin was nevertheless suppressed (< 1 mU/mL). The search for the JAK2 V617F gene mutation was positive and a diagnosis of polycythemia Vera (PV) was therefore made. In April 2011, treatment with HU 500mg daily was began, in combination with periodic phlebotomy. Therapy was well tolerated and the patient continued this treatment until February 2013, when he returned complaining for a new intense itching on hands and head. Physical examination showed several erythematous ulcerative lesions, sometimes being crusty, associated with scratching lesions and multiple actinic keratosis on a sun-damaged skin see Figure 1.
Figure 1.Erythematous ulcerative lesions with associated scratching lesionson hands (A) and multiple actinic keratosis on sun-damaged skin (B) (black arrows).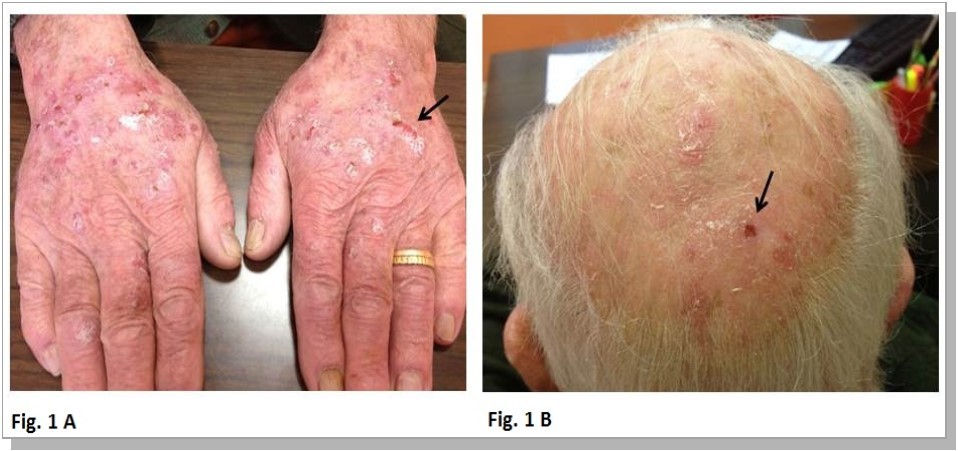
At that time the lab demonstrated that hematocrit was 45%, with normal white blood cells and platelets counts; markers of inflammation were absent; total serum IgE concentration was normal, which excluded an atopic diathesis. The dermatology Consultant confirmed the presence of ulcerated lesions partially scaling both on the back of the hands and on the head, which were considered as a possible manifestation of skin toxicity due to HU. The drug was therefore discontinued; the lesions improved in a few weeks, and three months after suspension of therapy the ulcerations of both hands and head were completely healed.
Discussion
Hydroxyurea is a cytostatic drug that is widely used to treat even elderly and frail patients with hematological diseases because of its safe toxicity profile. The epidemiology and incidence of the most significant HU-related toxicity have been recently reported in a retrospective study2. In this very large study, some form of mucocutaneuos toxicity has been observed in only a minority of subjects, 167 (4.8%) out of 3411 patients.
Mucocutaneous toxicity related to HU has been known since a long time, although severe toxic effects are generally unusual. A recent study from Latagliata et al., focusing on mucocutaneous HU-toxicity in a series of 993 patients, showed an incidence of 8.3% of HU-related mucocutaneuos adverse events3. The most frequent complications are diffuse hyperpigmentation, alopecia, melanonychia, onycholysis, dermatomyositis-like lesions and, at mucosal level, stomatitis and aphthous ulcers; all these phenomena usually present after a few months from the start of HU-treatment. The differential diagnosis of lesions due to HU-induced mucocutaneous toxicity includes atopic dermatitis, psoriasis, lichen planusand dermatomyositis4. Late cutaneous adverse events due to HU include ulcers and skin cancer; they usually occur after years of therapy and more frequently are localized on the legs. In the cohort of Latagliata et al, ulcerative lesions occurred in 3% of the patients, and were mostly localized in perimalleolar areas, with only 2 cases (0,2%) showing an involvement of photo-exposed areas. The ulcers appeared after a median period from initiation of HU treatment of 32.1 months, and in the large majority occurred in addition to other more common and early signs of mucocutaneous HU-toxicity3.
In the case here described, the patient presented a very unusual severe cutaneous toxicity, because ulcerative lesions appeared without previous mucosal manifestations and in absence of other more frequent forms of skin toxicity. Furthermore, in our case ulcers were localized only at the back of the hands and head, on sun-damaged skin, while no lesions were present on the legs.
Pathogenesis of skin ulcers after exposure to HU is not yet clear, but a direct cumulative cytotoxicity of HU on basal epithelial cells, resulting in atrophic skin lesions, has been proposed as a cause of such kind of skin damage5. In our case, the localization of ulcers in sun-exposed areas, also suggests the possible role of a photosensitivity to HU. This form of skin damage is very rarely seen after exposure to HU; to the best of our knowledge, only two previous cases of ulcers on sun-exposed areas such as heels, forearms and hands have been described6, 7. This aspect suggests the possibility of a cumulative damage given by the combination of HU and UV-stress. Recently the activity of HU on embryonic murine cells has been studied, showing that HU treatment induces the production of nitric oxide, which plays a central role in the rapid induction of apoptosis by p53 acetylation8. Treatment with HU may induce the progression of pre-malignant skin lesions to skin cancers , in particular in patients with chronic exposure to UV radiation and with many actinic keratoses.
The treatment of these complications in most cases requires the suspension of the drug, which leads to the complete healing of ulcers over several months, while the reduction in the dosage is often not sufficient to obtain a complete regression of lesions.
Conclusions
The case here reported indicates that mucocutaneous adverse events may occur even after years of treatment and that ulcerative lesions can appear also in the absence of other more frequent and early forms of mucocutaneous toxicity. Because the large use of HU for the treatment of an even larger population of patients, a particular attention has to be posed for the diagnosis of such unusual side effects, that require the interruption of the drug.
Consent
The patient has provided a full consent for its case to be anonymously described in a scientific case report
List of abbreviations
HU= Hydroxyurea ;
MPNs= myeloproliferative neoplasms ;
PV= polycythemia Vera.
References
- 1.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G. (2011) Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. , European LeukemiaNet.J Clin Oncol; 29, 761-770.
- 2.Antonioli E, Guglielmelli P, Pieri L, Finazzi M, Rumi E. (2012) AGIMM Investigators. Hydroxyurea-related toxicity in 3,411 patients with Ph’-negative MPN. , Am J Hematol 87, 552-554.
- 3.Latagliata R, Spadea A, Cedrone M, J Di Giandomenico, M De. (2012) Gruppo Laziale SMPC Ph1 neg. Symptomatic mucocutaneous toxicity of hydroxyurea in Philadelphia chromosome-negative myeloproliferative neoplasms: the Mister Hyde face of a safe drug.Cancer;. 118, 404-409.
- 4.Oskay T, Kutluay L, Ozyilkan O. (2002) Dermatomyositis-like eruption after long-term hydroxyurea therapy for polycythemia vera. , Eur J Dermatol; 12, 586-588.
- 5.Zargari O, Kimyai-Asadi A, Jafroodi M. (2004) Cutaneous adverse reactions to hydroxyurea in patients with intermediate thalassemia. , Pediatr Dermatol; 21, 633-635.
- 6.França E R, Teixeira M A, Matiaskde F, Antunes D E, Brazrde A. (2011) Cutaneous effects after prolongaded use of hydroxyurea in Polycythemia Vera. An Bras Dermatol;. 86, 751-754.
Cited by (1)
This article has been cited by 1 scholarly work according to:
Citing Articles:
Journal of Hematology and Oncology Research (2015) OpenAlex