Auricularia Polytricha (Mushroom) Regulates Testicular DNA Expression and Oxidative Stress Markers of Streptozotocin-Induced Diabetic Male Wistar Rat
Abstract
Local Nigerian men have been using AuriculariaPolytricha as a treatment for sexual dysfunction without supporting evidence from scientific experiments. This study was to investigate the effect of ethanolic extract of A. Polytricha on testicular DNA expression and some oxidative stress markers using STZ-Induced diabetic rats as a model. The experiment included six groups, Group A (Normal Control, treated with normal saline), Group B (treated with 65mg/kg.bw of STZ), Groups C, D, and E (treated with 250mg/kg.bw, 500mg/kg.bw, 1000mg/kg.bw AP after inducing diabetics), and Group F (treated with 40mg/kg.bw metformin after inducing diabetics). The experiment lasted for 35 days. After termination of the experiment, Fuelgen nuclear reaction was used for DNA demonstration to assess testicular DNA distribution while serum Superoxide Dimutase (SOD), Catalase and Melondialdehyde where evaluated using reagent based antioxidant enzyme assay. Results reveals that SOD and Melondialdehyde activities were remarkably (p<0.05) higher in diabetic control animals when compared with the normal control group. Values in Groups C, D and F that were administered with 250, 500mg/kg.bw A. polytricha and metformin respectively were also significantly (p<0.05) increased when compared with the normal control group. However, diabetic animals placed on 1000mg/kg.bw A. polytrichadid not show any statistical significance in comparison with normal control group but was remarkably (p<0.01) decreased when compared to the diabetic group that received low dose A. polytricha, an indication that the reversal is dose dependent. Catalase concentration in diabetic control animals was remarkably (p<0.05) higher when compared to the normal control but was not significantly (p<0.05) different in groups D (DM+500mg/kg.bw A. polytricha) and E (DM+1000mg/kg.bw A. polytricha) when compared with the normal control group. Diabetic control animals showed reduced magenta colour intensity of DNA and increased clustering and cross linking of DNA strands when compared with the normal control. However the degree of cross link in DNA strands was reduced in the diabetic animals placed on 1000mg/kg.bw A. polytrichawhen compared with the diabetic control group. Reversal in DNA damage and values of serum oxidative stress markers following administration of graded doses of A. polytricha could be attributed to essential phytochemical and therapeutic constituents in A. polytricha like polyphenol and flavonoid which can be found useful in prevention and treatment of diabetes induced testicular dysfunction. In summary, AP can contribute to a reversal in DNA damage and levels of serum oxidative stress markers in treating diabetes-induced testicular dysfunction.
Author Contributions
Academic Editor: Xiaoyong Lu, Department of Chemistry and Biochemistry, Ohio University, Athens, Ohio, 45701 USA.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Agbor Cyril Abang, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflicts of interest to declare.
Citation:
Introduction
Infertility is a very common complication in diabetic men. It is a recognized global health challenge in all countries, both developed and developing, with some couples requiring assisted reproductive interventions to be able to conceive. A good number of diabetic – related outcomes such as generation of oxidative stress and lipid peroxidation will negatively affect individuals with both type 1 and type 2 diabetes Mellitus in several ways such as progressive genotoxicity, structural defect in testicular tissue and significantly lower sperm quality 1
The major source of oxidative stress (OS) in most diseases including diabetes mellitus and also in normal physiological conditions is the mitochondria. During oxidative metabolism in the mitochondria, high concentration of oxygen is reduced to water, while part of oxygen though little is transformed to O2_ 2. Hyperglycemia can cause increased levels of Reactive Oxygen Specie (ROS) that is capable of resulting in cellular dysfunction and mutations 3.
Oxidative stress has been indirectly connected to the clinical consequences of sperm DNA damage 4. Oxidative DNA damage can occur through either oxidation of DNA bases primarily by direct attack on the purine and pyrimidine bases or through strand breaks and cross-linking in sperm DNA 5. In vitro induced oxidative stress significantly increases DNA fragmentation, modification in base structure, deletions, clustering and frame shifts in sperm chromatin 6.
Mitochondrial exposure to ROS provokes apoptotic process through release of Apoptosis Inducing Factor (AIF) which also cause DNA fragmentation. High level of ROS agitates mitochondrial membrane polarization and activates release of the cytochrome-C protein as a trigger of apoptosis 6.
The gradual shift to herbal therapy with its attendant increasing acceptance, even among the elite confirm the claim that herbal remedies can provide cure for several diseases, including infertility in men 7. A large number of plant and animal products have been tested for possible fertility regulatory properties 1.
Auricularia Polytricha (Mushroom) is one fungus found to have numerous medicinal properties. It belongs to the family Auriculariacaea and has been reported to have antioxidant property (Mau et al 2001), immunomodulatory activity 8, anti-tumour activity 9, anti-dementia properties 10, and hypocholesterolemic effect 11.
Auricularia Polytricha (AP) has been used locally by the Ejagham speaking people in Cross River State of Nigeria as an aphrodisiac agent in management and treatment of sexual dysfunction in men. This has been going on without the corresponding clinical trials and acceptable scientific experimentation to give credence to this age-long practice. This research is therefore intended to investigate the effect of ethanolic extract of Auricularia Polytricha AP (Mushroom) on testicular DNA expression and some oxidative stress markers of STZ-Induced diabetic rat model.
Materials and Methods
Preparation of Extract
Auricularia polytricha was obtained from Etomi central market located in Etung Local Government Area of Cross River State and taken to the department of biological sciences, University of Nigeria for identification. The mushroom was dried at room temperature, powdered and subjected to crude extraction with ethanol. Modified method was used for extraction. 200g of A. polytrichawas soaked in 1000ml of ethanol, labelled and covered for 72 hours, after which a clean filter paper (Watman No 1) was used to filter extracts. The filtrate was evaporated to dryness at 40o C in a vacuum using a rotatory evaporator. The extracts was weighed and kept at 40c in refrigerator until further use.
Ethical Clearance
Ethical clearance was obtained from the University of Calabar, Nigeria with Clearance Number: UC/FBMS/19/001291.
Experimental Animals
Thirty (30) adult male Wistar rats with average weight of 150g were used for this research. The rats will be kept in clean cages and divided into six groups designated A, B, C, D, E, and F with five rats in each group. The rats were allowed to acclimatize for two weeks in animal house, Department of Anatomy, Faculty of Medicine, University of Nigeria, Enugu Campus and allowed unrestricted access to commercially available chow (livestock feed) and water.
Experimental Design
(Table 1)
Table 1. Experimental animals was divided into six groups as follows:| S/N | Group | Designation | Treatment |
|---|---|---|---|
| 1 | A | Normal control | Distilled water |
| 2 | B | Diabetic control | 65mg/kg.bw of STZ |
| 3 | C | STZ + AP (Low Dose) | STZ + 250mg/kg.bw A. polytricha |
| 4 | D | STZ + AP (Mid Dose) | STZ + 500mg/kg.bw A. polytricha |
| 5 | E | STZ + AP (High Dose) | STZ + 1000mg/kg.bw A. polytricha |
| 6 | F | STZ + Metformin | STZ + 40mg/kg.bw Metformin |
Induction of Hyperglycaemia
After fasting for twelve hours, hyperglycaemia was induced by administering streptozotocin (STZ) intra-peritoneally. STZ was reconstituted in 0.5M Sodium citrate and administered at a dose of 65mg/kg.bw 13.
Confirmation of Diabetes
Diabetes was confirmed three days after administration of STZ using Accu-Check glucometer (Roche diagnostic, Germany) with blood samples obtained from tails of Wistar rats. Blood glucose levels (mmol/l) was checked before and after induction to ascertain hyperglycaemic state.
Administration of Extract
A. polytricha extract administration commenced two week after induction of hyperglycaemia by oral gastric intubation and lasted for 21 days. The experimental protocol was maintained for a total of 35 days.
Termination of Experiment and Collection of Samples for Analysis
At termination, the animals were sacrificed with the testes removed and plotted with filter paper. Both right and left testes were weighed together and then suspended in Bouins fluid for fixation, preparatory to histological processing. The sperm was collected by mincing from caudal epididymis with anatomical scissors into a glass beaker. 19 drops of diluting solution was added to dilute the semen in a proportion of 1:20. A sample from this homogenate was used for semen analysis.
DNA Demonstration
Fuelgen nuclear reaction was used for DNA demonstration (Fuelgen and Rossenbeck, 1924) to assess testicular DNA distribution.
Evaluation of Oxidative Stress Markers
Reagent based antioxidant enzyme assay was used to demonstrate for
Melondialdehyde (MDA)
Catalase
Soperoxide Dimutase (SOD)
Statistical Analysis
Quantitative data from this research was recoded and tabularized. Statistical significance of the differences between the groups was determined using one way analysis of variance (ANOVA) using SPSS statistical analysis program. P<0.05 will be considered significant.
Results
Oxidative stress markers (SOD, catalase and Melondialdehyde) activities of different experimental animals. Figure 1, Figure 2. Results from Fuelgen DNA demonstration is shown in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8.
Figure 1.Comparison of Supperoxide Dimutase (SOD) and Catalase in the different experimental groups.
Figure 2.Comparison of Melondialdehyde in the different experimental groups.
Figure 3.Normal Control (NC) X400 – Section of testis showing numerous deeply stained DNA with magenta color.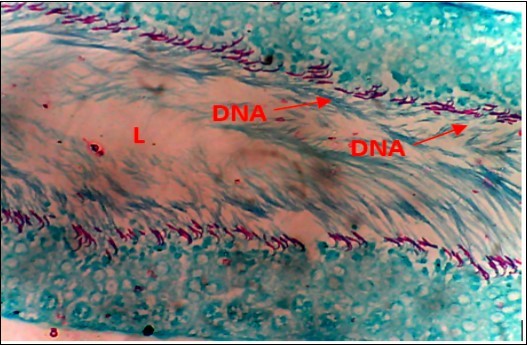
Figure 4.Diabetic Control (DC) X400 – Section of testis showing scanty DNA stained with magenta colour. DNA strands are cross linked and appear in clusters
Figure 5.DC+250mg/kg.bw of AP X400 – Section of testis showing scanty DNA deeply stained with magenta color. DNA strands are in clusters
Figure 6.(DC+500mg/kg.bw of AP) X400 – Section of testis showing scanty DNA deeply stained with magenta color. DNA strands are in clusters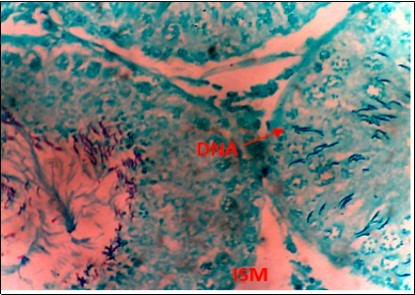
Figure 7.(DC+1000mg/kg.bw of AP) X400 – Section of testis showing scanty DNA deeply stained with magenta color. DNA strands has reduced clusters
Figure 8.(DC+Metformin) X400 – Section of testis showing deeply stained DNA with magenta color. DNA strands are higher clusters.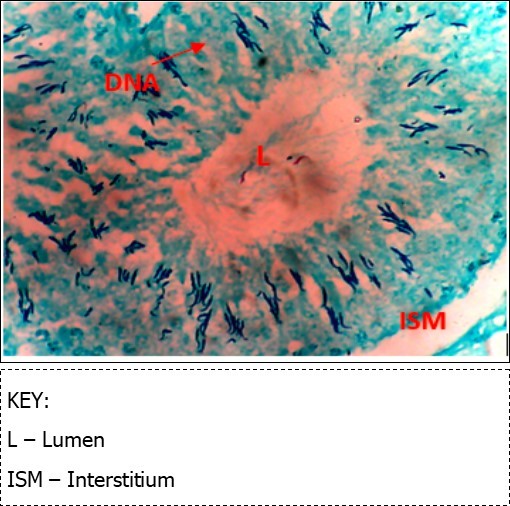
Discussion
Results obtained from analysis of serum oxidative stress markers shows that both SOD and Melondialdehyde activities were remarkably (p<0.05) higher in diabetic control animals when compared with the normal control group. Values in Groups C, D and F that were administered with 250, 500mg/kg.bw A. polytricha and metformin respectively were also significantly (p<0.05) increased when compared with the normal control group. However, diabetic animals placed on 1000mg/kg.bw A. polytrichadid not show any statistical significance in comparison with normal control group but was remarkably (p<0.01) decreased when compared to the diabetic group that received low dose A. polytricha, an indication that the reversal is dose dependent.
Catalase concentration in diabetic control animals was remarkably (p<0.05) higher when compared to the normal control but was not significantly (p<0.05) different in groups D (DM+500mg/kg.bw A. polytricha) and E (DM+1000mg/kg.bw A. polytricha)when compared with the normal control group. In a similar study, Mallidis et al., (2011) noted that melondialdehyde and caspase-3 activities were significantly higher, whereas SOD and GPx enzymatic activities were remarkably lower in diabetic rats when compared with normal control group. Three weeks administration of A. polytricha revealed a reduction in the values of melondialdehyde concentration in diabetic animal models. The decrease in these values was done in a dose-dependent manner. This findings are consistent with the work done by Mohasseb et al., 2011; Agbaje et al., 2007. They documented that antioxidant supplementation play a role on testicular germ cell apotosis of STZ-induced diabetic rat and can restore both testicular and systemic enzymatic activities of melondialdehyde and caspase-3. A. polytricha antioxidant property is achieved because of the presence of naturally occurring antioxidants like tocopherol, acobic acid, and total phenols 16. Sun et al., (2010) also reported that uronic acid has been fractionized from fruiting body of A. polytricha and that the higher the uronic acid content, the more effective the antioxidant activity of the polysaccharide. A. polytricha also has high phenolic and flavonoid content; glutathione reductase and superoxide dimutse activity 18. Chen et al. (2015) demonstrated that A. polytricha polysaccharides improved significantly, total antioxidant capacity and lipoprotein lipase activity in mice but was found to reduce melondialdehyde levels and arteriosclerosis index in rats and he attributed the strong antioxidant status to the phenolic compound in A. polytricha.
From findings in this study, irregular and distorted arrangements of DNA in all diabetic groups (Groups B, C, D, E and F) when compared with the normal control group, may have been due to displacement of sertoli cell within the germinal epithelium of seminiferous tubules. Strands of DNA were also seen arrange in clusters in diabetic groups, showing altered and defective structure which might have resulted from base free side deletion, frame shift, cross-linking and chromosomal rearrangement. The intensity of magenta colour development in Feulgen reaction for DNA demonstration was proportional to DNA concentration. There was reduced colour intensity in all diabetic groups (Groups B, C, D, E and F) when compared with the normal control. This is in line with report from Aitken and Krausz (2001). However the degree of distortion and cross-linking of DNA strand in the group of diabetic animal models placed on high dose (1000mg/kg.bw) of A. polytricha which may be a sign of amelioration. Groups C, D and F placed on 250mg/kg.bw A. polytricha, 500mg/kg.bw A. polytricha and standard anti-diabetic drug (metformin) respectively did not show remarkable differences in terms of DNA arrangements when compared with the diabetic control group. Groups D (500mg/kg.bw A. polytricha) and group E (1000mg/kg.bw) showed visible improvement in magenta colour intensity when compared with the diabetic control group.
Extensive experimental evidence 20, 21 have shown that ROS generated by diabetes is capable of modifying all bases, primarily guanine thereby altering the structure and producing defects by way of base free sites, deletion, shift in frame, DNA cross-link and chromosomal rearrangement. Agbaje et al., (2007) has documented that diabetic men are found to have a significantly higher percentage of spermatozoa and DNA damage and this was attributed to increased concentration of carboxymethyllysine (CML) and advanced glycation end (AGE) which are important triggers of oxidative stress in the reproductive tract of diabetic animals. DNA damage in this research may have resulted from increased generation of oxidative stress. Report from Mallidis et al., (2011) supports this findings.
The marginal reversal of DNA damage following 21 days of A. polytricha administration, as observed in this study may be linked to its polysaccharides constituent found to exhibit antimutagenic effect against in-vivo DNA damaging activities of indirectly acting alkylating agent. A. polytricha may have improved antioxidant status of experimental animals and was capable of ameliorating DNA damage which may be attributed to its potentials to regulate concentration of carboxymethyllysine (CML) and advanced glycation end (AGE) which are important triggers of oxidative stress in the reproductive tract of diabetic animals. AP is known to be a good exogenous source of antioxidant and may have ameliorated further DNA damage.
Extensive experimental research show that oxidative stress and reduced level of androgen hormone are potent risk factors associated with dysfunction in testes and impaired spermatogenic process in hyperglycaemic rat. Saleh el al, (2002) also showed that low concentration of ROS is required for sperm cells to acquire fertilization potentials and that, leukocytes and immature sperm cells will generate only but enough ROS necessary for the process of capacitation, acrosome reaction and possibly fusion, but excessive generation of ROS as in hyperglycaemic condition, without a corresponding balance in antioxidant production may have caused cellular damage and altered germinal epithelium seen in the diabetic animals. Substantial evidence implicates mitochondria as a major cellular organelle that generates ROS 24.
In conclusion, ethanolic extract of A. polytrichawas able to reverse damage in DNA and also mop up free radicals generated by prolonged hyperglycaemia in STZ-induced diabetic rat model. This reversal was in a dose dependent manner.
References
- 1.D K Bhatia, A K Sharma, P C, N C Khauduri. (2010) Antifertility effect of crude extract of Adiantum Lunulatum on Reproductve organs of male wister rats.Int. , journal on Biological Forum 2(2), 88-93.
- 2.M D Brand. (2010) The sites and topology of mitochondrial superoxide production. Experimental Gerontol,45,(7-8):. 466-472.
- 3.Nishikawa T, Edelstein D, Brownlee M. (2000) The missing link: a single unifying mechanism for diabetes complication. , The Kidney Journal 77, 526-530.
- 4.M A Baker, Krutskikh A, B J Curry, E A McLaughlin, R J Aitken. (2004) Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. , Boil reprod 71, 307-318.
- 5.Chabory E, Damon C, Lenoir A, Henry-Berger J. (2009) Epididymis seleno-independent glutathione peroxidase 5 maintain sperm DNA integrity in mice. , J Clin Invest 119, 2074-2085.
- 6.Maker K, Agarwal A, Sharma R. (2009) Oxidative stress and male infertility. , Indian Jmed Res 129, 357-367.
- 7.B O Anthony, A L Oladipo, K L Adedoyin, I A Tajudin. (2010) Phytochemistry and spermatogenic potential of aqueous extracts of Cissus Populnear. , The Science World Journal 6, 2140-2146.
- 8.Sheu F, Chien P J, Chien A L. (2004) Isolation and characterization of an immunomodulatory protein (APP) from the Jew’s Ear mushroom Auricularia polytricha. , Food Chem 87, 593-600.
- 9.Song G, Du Q. (2012) Structure characterization and antitumor activity of an α β-glucan polysaccharide fromAuriculariapolytricha.Food Research International45:. 381-387.
- 10.R N Bannett, F A Mellon, Foidl N, J H Pratt, M S Duport et al. (2013) Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multipurpose tree Moringa oleifera L. , Journal of Agricultural and Food Chemistry 51, 3346-3553.
- 11.Zhao C, Liao Z, Wu X. (2015) Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabetic mice. , J. Food Sci 79, 1002-1010.
- 12.Chen P, Yong Y, Gu Y.(2015).Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. , Int. J. Med. Mushrooms 17, 287-295.
- 13.N H Ugochukwu, N E Babady. (2003) Antihyperglycaemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin induced diabetic rat. , Life science 73(150), 1925-1938.
- 14.Mohasseb M, Ebied S, M A Yahia, Hussein N. (2011) Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rat. , Journal of Physiology and Biochemistry 67, 185-194.
- 15.Agbaje I, O’Neil J, Czerwiec A. (2009) Differences in rat model of diabetes mellitus in study of male reproduction. , International Journal of Andrology 33, 709-716.
- 16.Mau J L, Chao G R, Wu K T. (2001) Antioxidant properties of ethanolic extracts from several ear mushrooms. , J Agric Food Chem 49, 5461-5467.
- 17.Sun Y X, Liu J C, Kennedy J F. (2010) Purification, composition analysis and antioxidant activity of different polysaccharide conjugates (APPs) from the fruiting bodies of Auricularia polytricha. , Carbohydrate Polymers 82, 299-304.
- 18.Laios K, Karamanou M, Saridaki Z. (2012) Aretaeus of Cappadocia and first description of diabetes”. 11(1), 109-113.
- 19.Chen P, Yong Y, Gu Y.(2015).Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. , Int. J. Med. Mushrooms 17, 287-295.
- 20.R J Aitken, Krause C. (2001) Oxidative stress, DNA damage and the Y- chromosome. , Reproduction 122, 497-506.
- 21.Lenzi A, Cualosso F, Gandini L, Lombardo F, Dondero F. (1993) Placebo controlled, double-blind, cross-over trial of glutathione therapy in male infertility. , Human Reproduction 9, 2044-2050.
- 22.Mallidis C, Agbaje I, McClure N, Kliesh S. (2011) The influence of DM pn male reproductive function: a poorly investigated aspect of male infertility. , Journal of Urology A 50, 33-37.
