Abstract
Constrictive pericarditis (CP) represents a rare complication after heart transplantation (HTx), resulting from various postoperative events such as mediastinitis, pericardial effusion, or allograft rejection. We describe our recent experience with managing an HTx recipient who developed atypical patterns of CP predominantly involving the right ventricle. A 52-year-old male who had received heart transplantation 2.5 years before was admitted to our institution because of progressive symptoms of heart failure. The patient had experienced acute rejection twice post-HTx, both with International Society for Heart and Lung Transplantation grade 1R, undergoing an additional endomyocardial biopsy other than those performed during regular check-ups. On admission, echocardiography revealed paradoxical septal motion and a large cystic-like mass with a thick capsule in front of the right ventricle. Right heart catheterization revealed elevation of right atrial pressure, with severely reduced cardiac index. Magnetic resonance imaging revealed both seroma and a thick cystic-like capsule tightly adhered to the right ventricle. CP was suspected despite the atypical patterns of presentation. Seroma was removed through exploratory lateral thoracotomy, without improvement in symptoms, which was only achieved via subsequent pericardiectomy involving resection of the thickened parietal pericardium, removal of effusion fluid, and further excision of diffusely thickened visceral pericardium and epicardium. The patient is currently recovering uneventfully. The possibility of CP after HTx should be considered despite the rarity of this condition and HTx recipients should be closely monitored using various imaging modalities because CP typically demonstrates non-specific symptoms and physical findings of heart failure, with high mortality.
Author Contributions
Academic Editor: Hong-Jiang Wei, Yunnan Agricultural Univeristy
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Nobuhiko Ueda, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Constrictive pericarditis (CP) has been reported in recipients of heart transplantation (HTx).1 Various post-transplant complications such as mediastinitis, pericardial effusion including hemorrhage, and allograft rejection are considered as causes of post-transplant CP.2, 3, 4 While pericardiectomy is recognized as the fundamental treatment for CP, one-third of patients with CP have recurrence of heart failure symptoms after pericardiectomy.5
We describe herein our recent experience with managing a heart transplant recipient who had developed atypical patterns of CP predominantly involving the right ventricle.
Case Report
A 52-year-old male with a diagnosis of dilated-phase hypertrophic cardiomyopathy had a history of heart failure since the age of 41 years. After multiple episodes of hospitalization due to heart failure, the patient was registered as a candidate for HTx and underwent paracorporeal pulsatile-flow left ventricular assist device (LVAD) (Toyobo®; Nipro, Osaka Japan) implantation for bridge to transplantation at the age of 47 years. While waiting for HTx, the patient developed LVAD-related stroke with left hemiplegia. Three years after the LVAD implantation, the patient received orthotropic HTx. Immunosuppressive therapy was started according to a standard three-drug regimen including tacrolimus, mycophenolate mofetil, and prednisone, in combination with basiliximab as induction therapy. Endomyocardial biopsy (EMB) was performed ten times during the first year post-HTx, and by twice a year thereafter. The perioperative course mostly uneventful, but renal function gradually worsened. Therefore, at one year after HTx, immunosuppressive therapy was converted from the standard 3-drug regimen to everolimus with low-dose tacrolimus, in combination with low-dose prednisone. Following HTx, the patient experienced acute cellular rejection (ACR) twice, at 2 and 2.5 years postoperatively, both times with International Society for Heart and Lung Transplantation grade 1R.
At 2 years post-HTx, when the patient first developed ACR, echocardiography revealed mild pericardial effusion, but there was no deterioration in hemodynamic parameters compared with the findings noted on previous examinations. ACR was considered as the cause of mild pericardial effusion and the dose of immunosuppressive regimens were increased. Two months after that, pericardial effusion persisted even though resolution of ACR was confirmed on EMB. At 2.5 years post-HTx, echocardiography revealed paradoxical septal motion and a large cystic-like mass with a thick capsule adhered to the right ventricle (Figure 1a, b). Roughly from that time, the patient began to experience dyspnea on mild exertion and lower leg edema. Oral diuretics were added to the prescription to relieve symptoms but dyspnea and edema did not resolve entirely.
Figure 1.Echocardiography findings. A large cystic-like mass with a thick capsule was found in front of the the right ventricle (white arrows) (a. long axis view; b. short axis view). Paradoxical septal motion were seen in a motion mode image of left ventricle (c). Respiratory variation in both mitral (d) and tricuspid inflow velocities (e) were seen.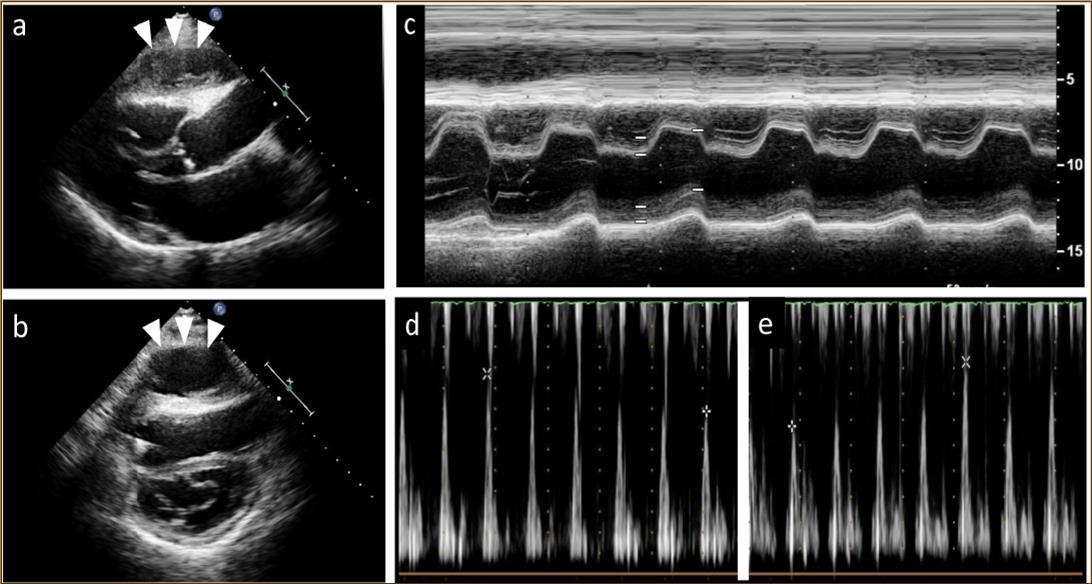
Figure 2.Magnetic resonance imaging findings. A thick cystic-like capsule which was tightly adhered to the right ventricle was seen (a. transverse view; b. sagittal view). A cystic-like capsule was removed after pericardiectomy (c. transverse view; d. sagittal view).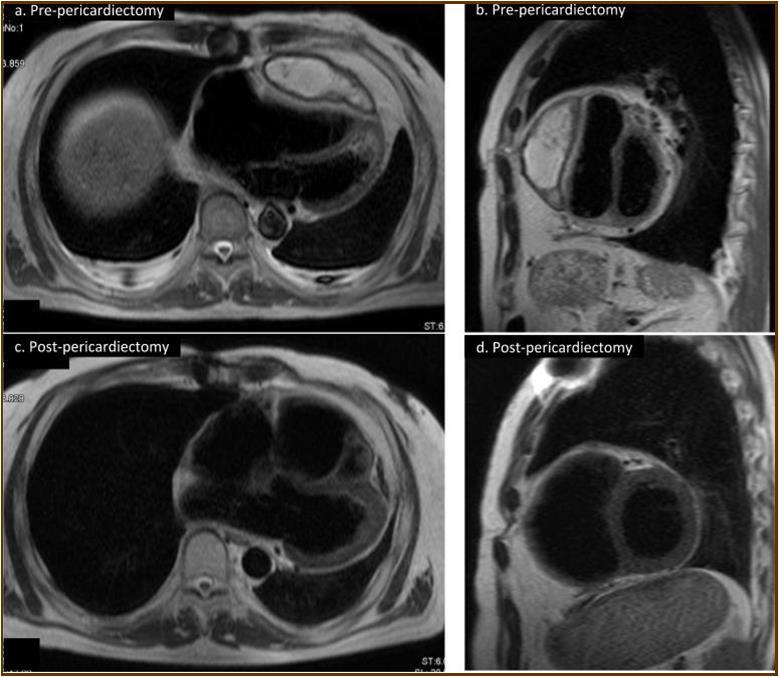
At the age of 52 years, the patient was admitted to our institution for both evaluation of heart failure and regular right heart catheterization including EMB. With respect to allograft rejection, ACR was again detected on regular EMB, and the immunosuppressive regimens were strengthened. Further echocardiographic investigations were conducted to explore the implications of findings indicative of constrictive physiology with thickened pericardium. Respiratory variation in both mitral and tricuspid inflow velocities was seen, but these findings did not meet the complete criteria for CP diagnosis (Figure 1c-e). Magnetic resonance imaging of the heart revealed seroma and a thick cystic-like capsule tightly adhered to the right ventricle (Figure 2a, b). Right heart catheterization revealed elevation of right atrial pressure with severely reduced cardiac index (Figure 3a). The classic dip-and-plateau pattern of right ventricular diastolic pressure and equalization of right atrial pressure and right ventricular diastolic pressure were also observed (Figure 3b). Based on these results, effusive CP or cardiac tamponade-like hemodynamics were strongly suspected, although the etiology remained unknown. Therefore, at 3 years post-HTx, the patient initially underwent removal of the seroma through exploratory lateral thoracotomy to elucidate the etiology of the disease. Yellowish fluid was found and the fluid was completely aspirated. Immunosuppressive regimens including everolimus, which may interfere wound healing, were temporarily converted to standard three-drug regimens. Removal of the seroma via lateral thoracotomy did not lead to improvement in symptoms or hemodynamics. Pathological analysis revealed fibrin and seroma, but no malignant cells or infection. Since the initial procedure did not have any effect on the patient’s symptom, pericardiectomy through median sternotomy was performed at two months after the initial procedure. Intraoperative findings indicated a thickened pericardium and effusion in front of the right ventricle. At first, after sternotomy and adhesiotomy, the tissue in front of the right ventricle, which appeared to be thickened parietal pericardium, was completely resected and the effusion fluid was removed. However, high central venous pressure (20 mmHg) persisted, resulting in a displacement of the left ventricle by the right ventricle. Therefore, additional tissue from the front of the right ventricle was excised, corresponding to diffusely thickened visceral pericardium and epicardium. After the procedure, central venous pressure decreased from 20 to 10 mmHg, and displacement of left ventricle resolved. Pathological analysis of the resected tissues demonstrated chronic fibrosis and chronic inflammation with plasma cells, but no infectious etiology was identified (Figure 4 a, b). After the pericardiectomy, hemodynamics (Figure 3a, c) and symptoms improved, and the patient is currently experiencing a favorable clinical course without complications.
Figure 3.Hemodynamic parameters. Both right and left ventricular filling pressure (RAP, and PCWP) were upregulated and CI was reduced just before pericardiactomy. RAP, and PCWP were lowered, and CI was upregulated after pericardiectomy. The classic dip-and-plateau pattern of right ventricular diastolic pressure was observed (b). CI, cardiac index; HTx, heart transplantation; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RHC, right heart catheterization; RVEDP, eight ventricular end-diastolic pressure; RVP, right ventricular pressure.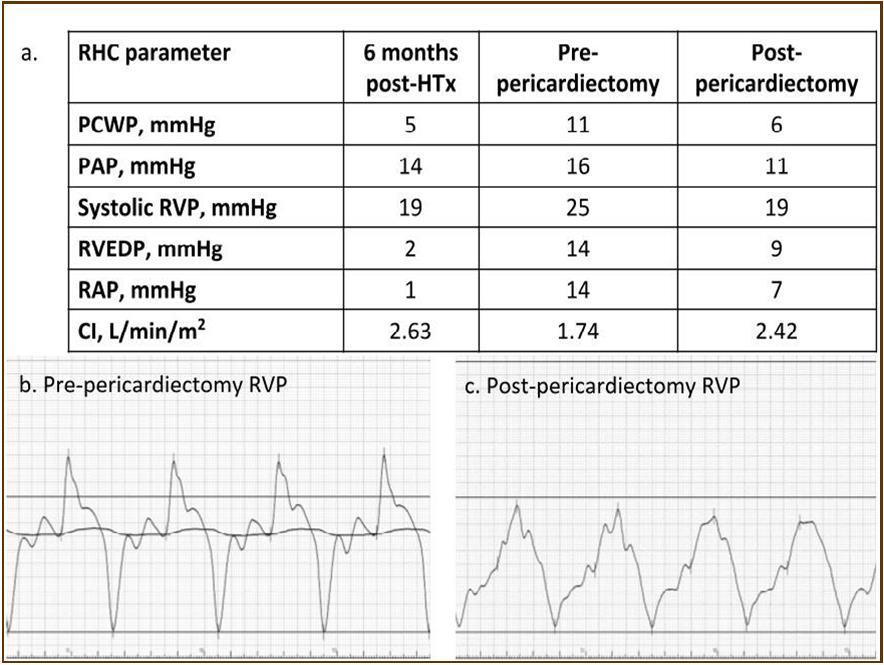
Figure 4.Pathology findings. Chronic fibrosis and chronic inflammation with plasma cells were seen, but no infectious etiology was identified in resected tissues (a. macroscopic; b. microscopic)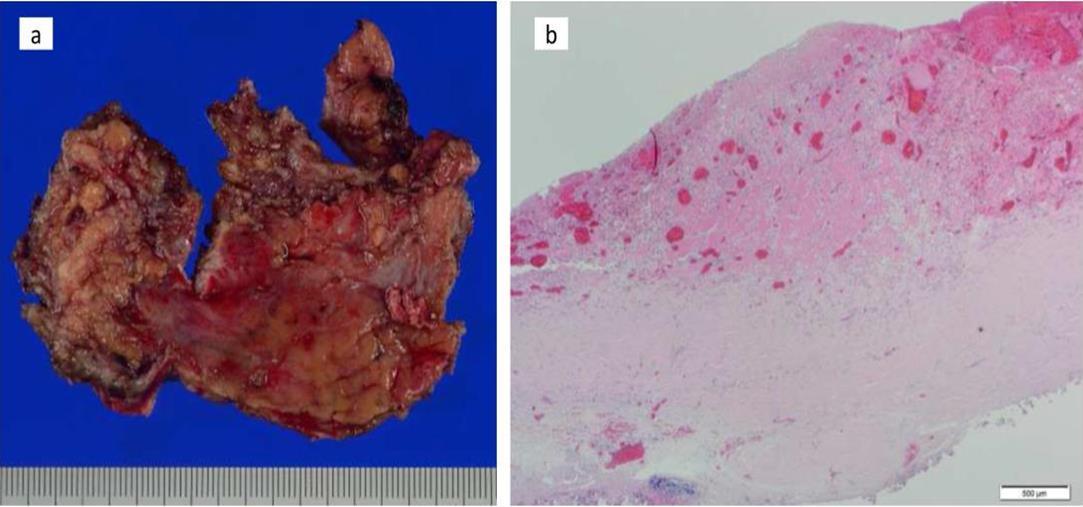
Discussion
Constrictive Pericarditis and Epicarditis after HTx
CP after HTx has been recognized as a rare disease, and moreover the diagnosis of CP is especially difficult in HTx recipients.3 A previous study reviewing 133 HTx recipients reported that 12% of patients developed postoperative complications involving the mediastinum and pericardium, and 2 patients (1.5%) developed signs of pericardial constriction.1 A more recent report reviewing 163 patients receiving pericardiectomy for CP reported that only 3 HTx recipients (1.8%) were found in this series.6 In our institution, 93 patients received HTx until 2016, and the patient described herein is the only one who underwent pericardiectomy for post-transplant CP (1.1%).
With regard to diagnosis, since patients with CP typically demonstrate non-specific symptoms and non-specific physical findings of heart failure, both right heart catheterization and various imaging investigations such as echocardiography, computed tomography, and magnetic resonance imaging should be performed. 7 For definitive diagnosis of CP, constrictive hemodynamic physiology together with thickened pericardium adhered to the myocardium should be proven.8 However, constrictive or restrictive physiology are sometimes non-specifically and temporary observed in HTx recipients for other reasons. In particular, ACR, which is one of the major causes of death during the early post-HTx period, has been reported to be associated with constrictive-restrictive physiology of the cardiac allograft.9 Seacord et al. reported that a heart transplant recipient with abnormal hemodynamics indicative of CP had exhibited improvement in the hemodynamic abnormality after ACR treatment with antilymphocyte globulin.10 ACR might promote constrictive-restrictive myocardial physiology, which improves upon resolution of ACR.9 Therefore, when the constrictive-restrictive physiology is suspected in HTx recipients, ACR should be initially ruled out, and accurate evaluation of hemodynamics should be performed to verify whether the findings fulfill the diagnostic criteria of CP.
In terms of etiology, various causes specific to HTx recipients should be taken into account in addition to conventional surgical matters. ACR with pericardial effusion and vasculopathy are characteristic causes of CP in HTx recipients.4,11 Further, administration of immunosuppressive agents in HTx recipients is known to double the incidence of mediastinitis or other intrathoracic infections postoperatively,12 which are thought to potentially cause CP in the early stage of after HTx in recipients with strong immunosuppression.2,3,13 In our patient, two episodes of ACR were detected before the development of CP, indicating that ACR might be the primary cause for CP in this patient. Since ACR is highly related to the development of pericardial effusion, repetitive ACR might have resulted in pericardial effusion followed by effusive CP. A previous study reviewing 15 patients with effusive CP reported that extensive epicardiectomy is the procedure of choice in patients requiring surgical intervention. Indeed, our patient also required not only parietal pericardiectomy but also visceral pericardiectomy including epicardiectomy.
The following three clinical features were notable in our patient: 1) effusive constrictive pericarditis, 2) the need for not only parietal pericardiectomy but also visceral pericardiectomy including epicardiectomy for resolving the constrictive hemodynamic physiology, and 3) right ventricle involvement. Considering the former two findings, we believe the etiology of CP in this patient is primarily related to ACR. However, right ventricle involvement is suggestive of a different etiology such as minor perforation by repeated EMB. Furthermore, since everolimus administration has been reported to be associated with the development of pericardial effusion, it is possible that everolimus administration had an additive effect on the effusive pathophysiology of CP.14
Conclusion
CP after HTx may have multiple etiologies so that its pathophysiology is usually complex and it is difficult to elucidate the exact mechanisms of the disease. Despite the fact that CP is a rare disease, transplant physicians should always keep in mind that CP may develop in HTx recipients with ACR, mediastinitis, and everolimus administration.15 Because recurrence of heart failure symptoms is often observed after pericardiectomy in patients with CP, and because the mortality rate of pericardiectomy is high, close follow-up is mandatory for the case described herein.
Disclosure
None of the authors declare any competing interests.
References
- 1.Carrier M, Hudo G, Paquet E, Leung T K, White M. (1994) Mediastinal and pericardial complications after heart transplantation. Not-so-unusual postoperative problems?. , Cardiovasc. Surg 2, 395-397.
- 2.Copeland J G, Riley J E, Fuller J. (1986) Pericardiectomy for effusive constrictive pericarditis after heart transplantation. , J. Heart. Transplant 5, 171-172.
- 3.Ramana R K, Gudmundsson G S, Maszak G J, Cho L, Lichtenberg R. (2005) Noninfectious constrictive pericarditis in a heart transplant recipient J. Heart Lung Transplant. 24, 95-98.
- 4.Hinkamp T J, Sullivan H J, Montoya A, Park S, Bartlett L et al. (1994) Chronic cardiac rejection masking as constrictive pericarditis Ann. , Thorac. Surg 57, 1579-1583.
- 5.Ling L H, Oh J K, Schaff H V, Danielson G K, Mahoney D W. (1999) Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy Circulation. 100, 1380-1386.
- 6.Bertog S C, Thambidorai S K, Parakh K, Schoenhagen P, Ozduran V. (2004) Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. , J. Am Coll Cardiol 43, 1445-1452.
- 7.Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G. (2015) ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). , Eur. Heart J 36, 2921-2964.
- 8.Verhaert D, Gabriel R S, Johnston D, Lytle B W, Desai M Y et al. (2010) The role of multimodality imaging in the management of pericardial disease Circ. , Cardiovasc Imaging 3, 333-343.
- 9.I A D'Cruz, Overton D H, Pai G M. (1992) Pericardial complications of cardiac surgery: emphasis on the diagnostic role of echocardiography. , J. Card. Surg 7, 257-268.
- 10.Seacord L M, Miller L W, Pennington D G, McBride L R, Kern M J. (1990) Reversal of constrictive/restrictive physiology with treatment of allograft rejection Am. , Heart J 120, 455-459.
- 11.Kumar R, Entrikin D W, Ntim W O, Carr J J, Kincaid E H. (2008) Constrictive pericarditis after cardiac transplantation: a case report and literature review J. Heart Lung Transplant. 27, 1158-1161.
- 12.S V Karwande, D G Renlund, S L Olsen, W A Gay, W E Richenbacher. (1992) . , Ann. Thorac. Surg 54, 1039-1045.
- 13.Roca J, Manito N, Castells E, Gómez Hospital JA, Miralles A. (1995) Constrictive pericarditis after heart transplantation: report of two cases. , J. Heart Lung Transplant 14, 1006-1010.
