Abstract
The aim of this study was to assess the use of ambulatory acoustic cardiography during the initial data collection of the longitudinal study of a rural population in Switzerland (n=297, mean age 48.9 ±16.5 years, 57% female). Ambulatory acoustic cardiography non-invasively can assess sleep disordered breathing (SDB) and provides markers of left ventricular systolic and diastolic dysfunction. The percentage of the third heart sound detected during sleep decreased significantly across age groups (age < 40 years, 40-60 years, > 60 years) for both genders (males, p=0.04; females, p=0.02). The percentage of a fourth heart sound detected exhibited an increasing trend for both genders with age suggesting increased diastolic dysfunction with aging. Mean electromechanical activation time (EMAT) during sleep was within the normal range across age groups and both genders (male 93.7 ± 11.6 ms, female 94.6 ± 13.0 ms), and did not vary significantly with age. A large proportion of subjects had a high likelihood of sleep disordered breathing (17.6%). Baseline characteristics categorized by SDB severity indicate increasing age, male gender and being overweight (BMI ≥ 25) to be associated with greater SDB severity. Acoustic cardiography findings categorized by SDB severity reveal increased nocturnal non-dipping heart rate, presence of atrial fibrillation, prolonged QRS duration and QTc interval, increased percentage of fourth heart sound detected, and longer EMAT to be significantly associated with greater SDB severity. Overall, acoustic cardiography detected a very low prevalence of systolic dysfunction, age-related increases in diastolic dysfunction and a moderate prevalence of sleep disordered breathing.
Author Contributions
Academic Editor: Osmar Centurion, Professor of Medicine. Asuncion National University. Cardiology Division. First Department of Internal Medicine. Asuncion. Paraguay, Email: [email protected]
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Peter Bauer, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The life expectancy in Switzerland is particularly high and due, in part, to nationwide patient management strategies aimed at lowering blood pressure 1 and controlling hyperlipidemia to decrease the number of deaths due to acute coronary disease 2 and stroke, but cardiovascular disease remains the leading cause of mortality and morbidity. The longitudinal SWICOS cohort study 3 is intended to examine and longitudinally follow health status and disease risk factors in select rural Swiss populations to support development of new implementable and successful preventive strategies for healthy ageing. In addition to conventional epidemiological parameters of cardiovascular health, SWICOS uses contemporary technologies including ambulatory acoustic cardiography monitoring 4 as part of the establishment of a well-defined database of health status.
Acoustic cardiography evaluation involves a 24-hour ambulatory Holter-type recording performed using a device that stores continuous ECG, heart sound and respiratory data (AUDICOR, Inovise Medical, Inc., Beaverton, Oregon, USA). Analysis of the acoustic cardiography recording results in parameters to evaluate diastolic function, in particular, the presence of a fourth heart sound (S4), which has been shown to be associated with increased left ventricular stiffness 5. Acoustic cardiography also produces parameters to assess systolic function including the presence of a third heart sound (S3) shown to be correlated with elevated filling pressure 6, electromechanical activation time (EMAT) that reflects the time required for the left ventricle to generate enough pressure to close the mitral valve 7 and the Systolic Dysfunction Index (SDI, a composite score of ECG and heart sound parameters). In addition, ambulatory monitoring with acoustic cardiography produces an automated measure of the severity of sleep-disordered breathing 8.
Results from ambulatory acoustic cardiography have previously been reported on small asymptomatic 9 and heart failure populations 10. Lausanne, Switzerland has been the setting for a population-based survey of cardiovascular risk factors (CoLaus) 11 and, separately, at-home sleep disordered breathing assessment (HypnoLaus) 12. However, little data exists on the associations between sleep disordered breathing and left ventricular systolic / diastolic function within a rural Swiss population.
The aim of this study was to assess the applicability of the use of acoustic cardiography in voluntary participants of the recently begun longitudinal study of a rural population in Switzerland. In this paper we report only the acoustic cardiography findings for the initial data collection to evaluate systolic and diastolic heart function and sleep disorders.
Materials and Methods
Ambulatory acoustic cardiographic Holter data were collected between April, 2015 and January, 2017 from residents within the region of Cama, Switzerland age 15 years and older that were willing to participate long-term in the SWICOS study. There were no exclusion criteria. The study received approval from the ethics committee of Nordwest und Zentralschweiz (reference number EKNZ 2014-209; NCT02282748).
Informed consent was obtained from all subjects. Anamnestic data was obtained through questionnaire and interview of the subject. Patient height and weight were measured during initial examination.
Acoustic Cardiographic Holter Recordings
The ambulatory acoustic cardiography recording was obtained by placing standard ECG electrodes in modified limb locations and dual-purpose ECG / sound AUDICOR sensors placed in either the standard V4 position or both the V3 and V4 precordial positions while the subject was in a supine position (Figure 1). The main AUDICOR recording device was secured on the upper chest wall using two standard ECG snap-type electrodes. Adequate quality of the ECG and heart sound signals was confirmed visually by study personnel before starting the recording. Simultaneous ECG, heart sound and respiration data were recorded simultaneously for the duration of the recording and partitioned into awake and sleep periods.
Figure 1.Acoustic cardiography monitoring device, sensor and placement on torso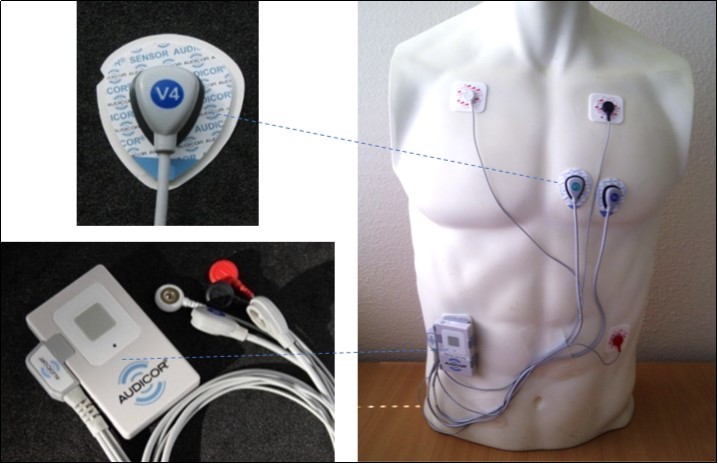
The ECG signals were overread by trained Holter technicians for ectopy and rhythm abnormalities. Automated analysis of the ECG data produced results for QRS duration, QTc interval and heart rate.
The ECG and heart sound data underwent automated analysis for the presence of diastolic heart sounds (S3 and S4 Strength), electromechanical activation time (EMAT), electromechanical activation time divided by the cardiac cycle interval (EMATc) and the Systolic Dysfunction Index (SDI). The AUDICOR automated heart sound algorithm is a hidden Markov model with inputs derived from a 6-level stationary wavelet transformation of the acoustic signal and from select fiducial times of the simultaneous ECG signal. The output includes a segmentation of the acoustic signal into individual heart sounds, as well as measurements derived from that segmentation. The S3 and S4 strength parameters are a combination of intensity and persistence of the acoustic signal within the segments appropriate in timing for those heart sounds. S3 / S4 Strength is expressed on a 0 to 10 scale where if the strength is ≥ 5.0 a third or fourth heart sound is considered present. EMAT is the interval from Q onset to the mitral component of the first heart sound expressed in msec, and is prolonged in systolic heart failure 4. EMATc is equal to EMAT divided by the length of the cardiac cycle and is expressed as a percentage with values ≥15% indicating poor prognosis from left ventricular systolic dysfunction 13. Acoustic cardiography produces the systolic dysfunction index (SDI) which is non-linear combination of QRS duration, QR interval, S3 strength and EMATc and is mapped to a continuous scale of 0 to 10. The multiparameter score SDI is expressed on a 0 to 10 scale and when ≥ 5.0 indicates left ventricular systolic dysfunction and when ≥ 7.5 indicates severe left ventricular systolic dysfunction with elevated filling pressure.
The acoustic cardiographic analysis for ECG is similar to standard Holter and encompasses the entire recording while the analysis of heart sounds and sleep-disordered breathing occurs during sleep hours. Results from the analysis during sleep include the percentage of third and fourth heart sounds detected (% S3 / S4 Strength ≥ 5.0), the average value of EMAT (msec), percentage of EMATc ≥ 15 (%), percentage of SDI ≥ 5 (%), and percentage of SDI ≥ 7.5 (%).
Signals from the AUDICOR triaxial accelerometer located on the chest wall are bandpass filtered and then undergo further signal processing to create a pseudo-respiration signal that is used to detect individual sleep-disordered breathing events (i.e., apneas and hypopneas). The timing and duration of individual SDB events are used to create a sleep disordered breathing severity index (events per hour). Apnea and hypopnea events greater than ten seconds in duration that meet certain physiological timing constraints and occur during quiescent periods are considered valid SDB events. These are totaled and divided by total sleep time to create the SDB severity index. The SDB algorithm was developed on learn portions of large databases from subjects undergoing overnight polysomnography (PSG). The PSG data was collected, annotated by trained experts for episodes of sleep disordered breathing, and scored in accordance with American Association of Sleep Medicine (AASM) 2007 standards including generation of the apnea / hypopnea index (AHI). The AUDICOR sleep disordered severity index was developed to correlate with the PSG AHI and categorize subjects into groups of borderline, low probability and probable likelihood. Dillier et al. 8 published performance results for the AUDICOR SDB severity index against the AASM thresholds that uses AHI to classify mild, moderate and severe sleep apnea (mild, 5 ≤ AHI < 15; moderate, 15 ≤ AHI < 30; severe, AHI ≥ 30). In addition, the sound data allows the detection of snore events distinguished as period, high frequency signals that are used to determine the percentage of sleep time with snore events. This parameter was validated against PSG snore events 8.
The clinical performance of all the parameters obtained through acoustic cardiography by the AUDICOR device have been tested and validated in previous invasive and non-invasive studies with independent clinical correlates (e.g., cardiac catheterization, pressure / volume loops, echocardiography, overnight polysomnography) 5, 6, 7, 8, 9, 10.
Statistical Analysis
The results are presented as percentages for categorical variables and analyzed using the Pearsonchi-square test or Fisher’s exact test as appropriate. Continuous normally distributed variables are expressed as means ± 1 standard deviation (SD) and compared using the Student’s two-tailed unpaired t-test. To test for differences among groups one-way ANOVA was used. Continuous non-normally distributed variables are expressed as medians and interquartile ranges and analyzed using the Mann-Whitney U test or Kruskal–Wallis as appropriate. P values < 0.05 to be considered statistically significant. The statistics software used for analysis was IBM SPSS Statistics (version 23, IBM Corp. Armonk, NY, USA).
Results
A total of 307 subjects from one location in Switzerland were enrolled. There were 10 subjects excluded from the acoustic cardiography analysis due to poor signal quality. Of the 297 subjects with suitable acoustic cardiography recordings, 284 included the sleep period and had at least 4 hours of sleep necessary to assess sleep-disordered breathing.
The baseline characteristics of the 297 subjects (female 57%) with ambulatory acoustic cardiography recordings (male age range 16 to 89 years; female age range 15 to 87 years) are presented in Table 1, for all ages and within the age categories used in further analyses (age < 40, 40 to 60, and > 60 years). As expected, age over 40 years was associated with significantly higher prevalence of hypertension, hyperlipidemia, increased BMI, coronary artery disease, myocardial infarction and heart failure.
Table 1. Baseline Characteristics, all subjects and by age group| Total | Age groups | |||||||
| < 40 y | 40-60 y | >60y | ^ P | * P | ** P | # P | ||
| N= 297 | N=72 | N=150 | N=75 | |||||
| Age (years) | 48.9 ±16.5 | 26.4 ± 7.2 | 49.3 ±5.4 | 69.8 ±6.8 | <0.001 | <0.001 | <0.001 | <0.001 |
| Female | 169/297 (56.9) | 39/72 (54.2) | 92/150 (61.3) | 38/75 (50.7) | 0.65 | 0.33 | 0.74 | 0.15 |
| Hypertension~ | 54/293 (18.4) | 0/71 | 22/147 (15.0) | 32/75 (42.7) | <0.001 | <0.001 | <0.001 | <0.001 |
| Hyperlipidemia~ | 54/293 (18.4) | 2/72 (2.8) | 18/148 (12.2) | 34/73 (46.6) | <0.001 | <0.001 | <0.001 | <0.001 |
| Smoking (current)~ | 54/295 (18.3) | 21/72 (29.2) | 25/150 (16.7) | 8/73 (11.0) | 0.030 | 0.35 | 0.007 | 0.32 |
| Smoking (former)~ | 67/295 (22.7) | 9/72 (12.5) | 32/150 (21.3) | 26/73 (35.6) | 0.030 | 0.14 | 0.002 | 0.036 |
| BMI (kg / m2) | 25.6 ± 7.2 | 23.1 ± 4.1 | 26.0 ± 9.0 | 27.3 ± 4.6 | 0.008 | 0.009 | <0.001 | 0.24 |
| Overweight, BMI ≥25 | 148/297 (49.8) | 24/72 (33.3) | 74/150 (49.3) | 50/75 (66.7) | <0.001 | 0.030 | <0.001 | 0.016 |
| Obese, BMI ≥30 | 45/297 (15.2) | 4/72 (5.6) | 20/150 (13.3) | 21/75 (28.0) | 0.001 | 0.11 | <0.001 | 0.010 |
| Diabetes | 12/294 (4.1) | 0/72 | 3/149 (2.0) | 9/73 (12.3) | <0.001 | 0.55 | 0.003 | 0.003 |
| CAD | 9/294 (3.1) | 0/72 | 2/148 (1.4) | 7/74 (9.5) | 0.001 | 1.00 | 0.013 | 0.007 |
| Myocardial infarction | 9/295 (3.1) | 0/71 | 1/149 (0.7) | 8/75 (10.7) | <0.001 | 1.00 | 0.007 | 0.001 |
| Heart failure | 12/294 (4.1) | 0/71 | 4/149 (2.7) | 8/74 (10.8) | 0.002 | 0.31 | 0.006 | 0.022 |
| Stroke | 3/294 (1.0) | 0/71 | 1/149 (0.7) | 2/74 (2.7) | 0.10 | 1.00 | 0.50 | 0.26 |
| Asthma | 33/292 (11.3) | 7/71 (9.9) | 18/148 (12.2) | 8/73 (11.0) | 0.87 | 0.82 | 1.00 | 1.00 |
There were no significant differences in mean sleep time between males and females, or across age ranges (mean sleep time: all 4.9 ± 1.5, males 4.7 ± 1.4, females 5.0 ± 1.5 hours).
In aggregate, females had a significantly higher heart rate during sleep than males (62.2 ± 8.3 vs. 58.8 ± 8.9 bpm, p=0.018) and also significantly higher heart rate during wake than males (77.7 ± 8.8 vs. 74.4 ± 11.5 bpm, p=0.005), Figure 2. Within age groups, only the 40-60 year group had a significant difference in heart rate across gender (sleep p=0.02; awake p=0.007). Nocturnal non-dipping of heart rate was defined as sleep / awake heart rate ratio greater than 0.90 14. Among 287 subjects, 30 (10.5%) had nocturnal non-dipping of heart rate (Figure 3; males 13.8%, females 7.9%). The subjects with nocturnal non-dipping of heart rate were on average 4 years older than those without (52.4 ± 16 vs. 48.5 ± 16 years), with more diabetes, hypertension, higher BMI and they were less frequently current smokers. However, none of these reached statistical significance.
Figure 2.Mean awake and sleep heart rate for males and females
Figure 3.Nocturnal non-dipping of heart rate for males and females
The percentage of S3 detected during sleep decreased significantly across age groups for both males and females (males p=0.04, females p=0.02), and differed significantly between males and females only in the 40 to 60 year old group (p=0.02), Figure 4. For ages > 40 years, the mean values of the percentage of S3 detected were 4% or less. In contrast, the percentage of S4 detected exhibited an increasing trend with age for both males and females; however, only females had a significant difference across age groups (females p=0.04, males p=0.09), Figure 4.
Figure 4.Diastolic third and fourth heart sounds, percentage detected during sleep
Mean electromechanical activation time during sleep was less than 100 msec across age groups and both genders (male 93.7 ± 11.6 ms, female 94.6 ± 13.0 ms), and did not vary significantly (Figure 5). The percentage of EMATc ≥ 15 during sleep, Figure 5, was very low with a median of 0.2% (IQR 0.04, 0.94%). Males had a significant difference in %EMATc ≥ 15 during sleep across age groups while females did not (males p=0.01, females p=0.50) although the absolute percentage did not suggest LV systolic dysfunction.
Figure 5.Mean electromechanical activation time (EMAT) and percentage of EMATc ≥15 during sleep
The percentage of SDI ≥ 5.0 and percentage of SDI ≥ 7.5 during sleep was very low with medians of 0.06% (IQR 0, 0.3%) and 0% (IQR 0, 0.07%), respectively (Figure 6).
Figure 6.Percentage of SDI ≥ 5.0 and SDI ≥ 7.5 during sleep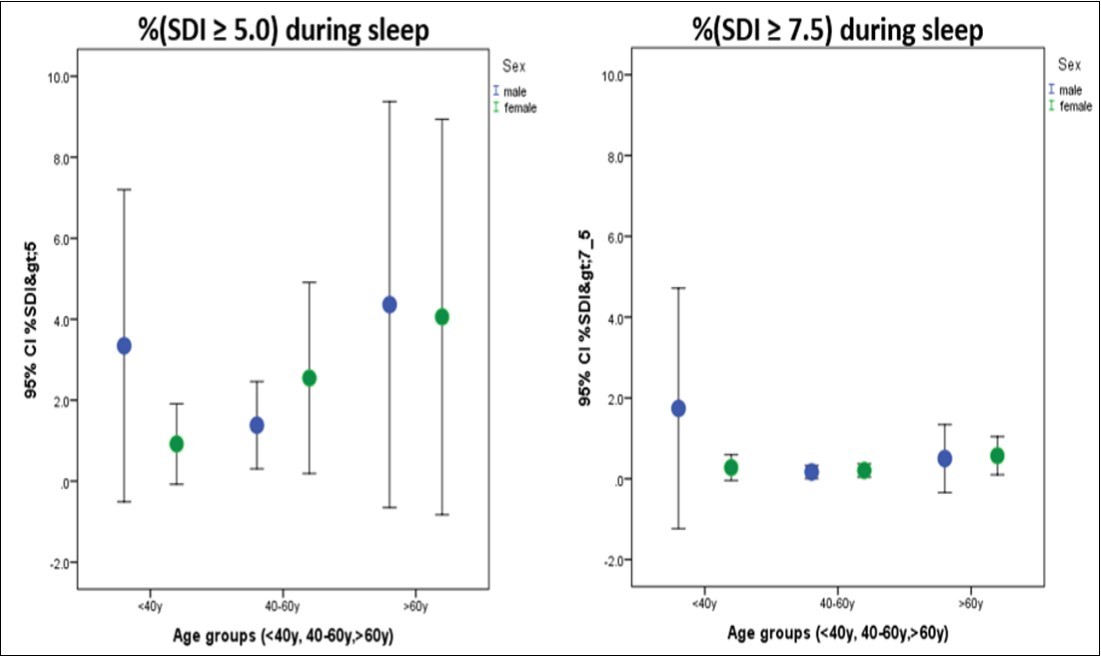
While both males and females had increasing sleep-disordered breathing severity trends with age, only female SDB trends with aging reached statistical significance (males p=0.59, females p=0.05), Figure 7. The sleep-disordered breathing severity index is used in the acoustic cardiography report to produce statements concerning the likelihood of SDB as low probability, borderline and probable. Baseline characteristics categorized by SDB likelihood statement indicate increasing age, male gender and being overweight (BMI ≥ 25) to be associated with greater SDB severity (Table 2). Within the highest severity SDB group, 66% of the population were either overweight or obese. ECG findings categorized by SDB likelihood statement reveal increased nocturnal non-dipping heart rate, presence of atrial fibrillation, prolonged QRS duration and QTc interval to be increased with greater SDB severity (Table 3). Acoustic cardiography findings categorized by SDB likelihood show increased percentage of S4, prolonged EMAT and EMATc ≥ 15% to be significantly associated with greater SDB severity. The percentage of time spent snoring is also increased with greater SDB severity suggesting the disorder to be obstructive rather than central in nature.
Figure 7.Sleep-disordered breathing severity index during sleep for males and females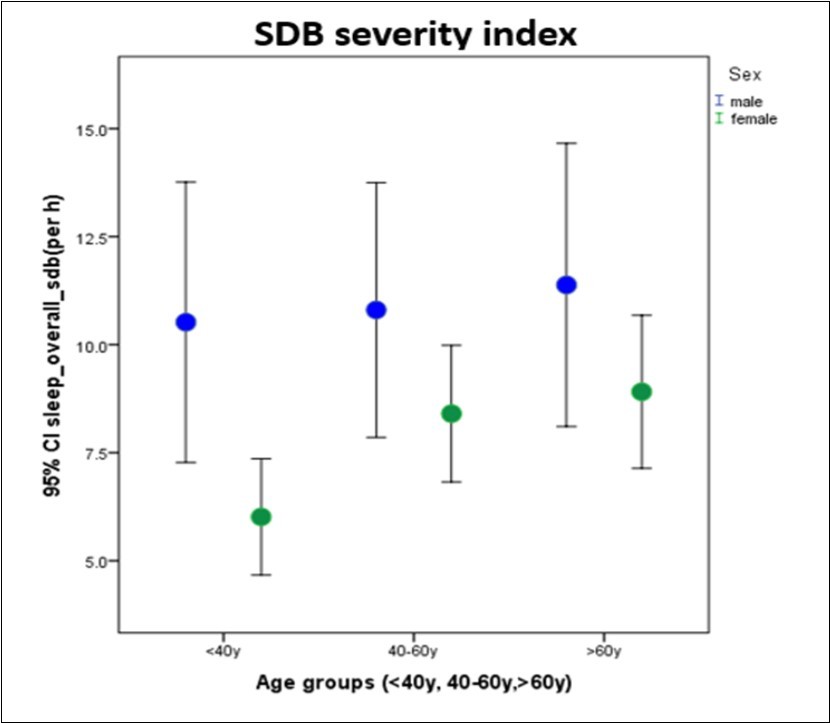
| Sleep-disordered breathing findings | P values | ||||||
| low probability | borderline | probable | ^ P | * P | ** P | # P | |
| N=214 | N= 20 | N=50 | |||||
| Age (years) | 47.6 ± 16.6 | 50.2 ± 13.1 | 53.6 ± 17.4 | 0.068 | 0.49 | 0.024 | 0.44 |
| Female | 133/214 (62.1) | 9/20 (45.0) | 20/50 (40.0) | 0.009 | 0.15 | 0.06 | 0.79 |
| Hypertension~ | 35/211 (16.6) | 3/20 (15.0) | 14/50 (28.0) | 0.19 | 1.00 | 0.072 | 0.36 |
| Hyperlipidemia~ | 35/213 (16.4) | 2/20 (10.0) | 15/49 (30.6) | 0.055 | 0.75 | 0.042 | 0.12 |
| Smoking (current)~ | 40/214 (18.7) | 5/19 (23.3) | 9/49 (18.4) | 0.29 | 0.25 | 1.00 | 0.51 |
| BMI (kg / m2) | 25.3 ± 8.0 | 26.9 ± 4.9 | 26.4 ± 4.5 | 0.44 | 0.37 | 0.34 | 0.68 |
| Overweight, BMI ≥25 | 92/214 (43.0) | 14/20 (70.0) | 23/50 (46.0) | 0.02 | 0.032 | 0.004 | 1.00 |
| Obese, BMI ≥30 | 27/214 (12.6) | 5/20 (25.0) | 10/50 (20.0) | 0.20 | 0.16 | 0.18 | 0.75 |
| Diabetes | 7/212 (3.3) | 2/20 (10.0) | 2/50 (4.0) | 0.45 | 0.18 | 0.68 | 0.57 |
| CAD | 6/214 (2.8) | 0/20 | 3/50 (6.0) | 0.30 | 1.00 | 0.38 | 0.55 |
| Myocardial infarction | 3/213 (1.4) | 1/20 (5.0) | 4/50 (8.0) | 0.065 | 0.30 | 0.026 | 1.00 |
| Heart failure | 9/213 (4.2) | 0/20 | 3/50 (6.0) | 0.36 | 1.00 | 0.71 | 0.55 |
| Stroke | 2/212 (0.9) | 0/20 | 1/50 (2.0) | 0.67 | 1.00 | 0.47 | 1.00 |
| Asthma | 29/210 (13.9) | 1/20 (5.0) | 3/50 (6.0) | 0.15 | 0.49 | 0.16 | 1.00 |
| Sleep-disordered findings | P values | ||||||
| low probability | borderline | probable | ^ P | * P | ** P | # P | |
| AUDICOR findings | N=214 | N= 20 | N=50 | ||||
| SDB severity index (events / hour) | 1.0 ± 0.5 | 3.6 ± 0.7 | 7.8 ± 1.9 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| % Time snoring (%) | 4.3 ± 9.8 | 5.2 ± 9.7 | 12.4 ± 17.4 | < 0.001 | 0.70 | 0.002 | 0.03 |
| Heart rate, awake (bpm) | 76.8 ± 9.8 | 77.9 ± 12.1 | 73.3 ± 9.7 | 0.057 | 0.65 | 0.021 | 0.097 |
| Heart rate, sleep (bpm) | 61.2 ± 7.9 | 64.4 ± 11.1 | 60.0 ± 8.9 | 0.13 | 0.092 | 0.34 | 0.085 |
| Heart rate ratio (sleep/awake) | 0.80 ± 0.07 | 0.83 ± 0.06 | 0.82 ± 0.09 | 0.046 | 0.072 | 0.047 | 0.77 |
| Nocturnal non-dipping heart rate (%) | 16/214 (7.5) | 2/20 (10.0) | 11/50 (22.0) | 0.020 | 0.66 | 0.007 | 0.25 |
| Atrial fibrillation present | 1/214 (0.5) | 0/20 | 3/50 (6.0) | 0.037 | 1.00 | 0.022 | 0.55 |
| AV block present | 10/214 (4.7) | 1/20 (5.0) | 3/50 (6.0) | 0.93 | 1.00 | 0.72 | 1.00 |
| QRSd (ms) | 101 ± 11 | 110 ± 26 | 110 ± 18 | 0.008 | 0.04 | <0.001 | 1.00 |
| QTc (ms) | 427 ± 17 | 427 ± 19 | 435 ± 22 | 0.040 | 0.99 | 0.012 | 0.20 |
| % S3 detected, median (IQR) | 0.4 (0.1; 2.6) | 0.1 (0.1; 0.8) | 0.2 (0.0; 0.8) | 0.027 | 0.13 | 0.017 | 0.88 |
| % S4 detected, median (IQR) | 7.3 (0.6; 31.8) | 4.7 (1.4; 21.7) | 17.9 (3.8;34.0) | 0.018 | 0.58 | 0.15 | 0.087 |
| EMAT (ms), median (IQR) | 94 (86, 100) | 90, (82.5, 101) | 99 (88, 106) | 0.014 | 0.98 | 0.02 | 0.18 |
| % EMATc>15, median (IQR) | 0.1 (0.0; 0.8) | 0.6 (0.1; 3.8) | 0.4 (0.1; 1.6) | 0.01 | 0.003 | 0.003 | 0.33 |
Discussion
The results provided in this paper represent the acoustic cardiography results from the initial data collection (n=297) of the longitudinal SWICOS study conducted in the region of Cama, Switzerland. Recent studies have shown that both short (typically < 5-6 hours) and long (typically >8-9 hours) durations of sleep are associated with increased mortality, obesity and risk of CVD. The mean sleep time (4.9 ± 1.5 hours) for our population would be regarded as "short" in duration with no significant differences related to gender or aging. Cappuccio et al. have shown that short sleep duration is associated with greater risk of developing or dying of coronary heart disease or stroke 15, and Ford 16 reported increased 10-year risk of CVD with short sleep duration (14.5%). Our results suggest the need for lifestyle modifications and public education to improve sleep duration to reduce cardiovascular risk.
Although considered physiologic in those under 40 years, the presence of a diastolic third heart sound has been shown to identify patients with more advanced heart failure 17 and has been associated with adverse outcomes in both heart failure and acute myocardial infarction 18, 19. The S3 occurs in diastole during early passive filling and is caused by the sudden deceleration of rapid filling into a distended or stiff ventricle 20. Using acoustic cardiography on short recordings, detection of a third heart sound has been correlated to elevated LV end-diastolic pressure and reduced ejection fraction 21, and in emergency department patients with signs or symptoms of acute decompensated heart failure has been found to be specific for primary heart failure 22. We found a decrease in the percentage of S3 detected during sleep after age 40 years with a mean of 4% or less detected after age 40 years similar to the results of Dillier et al. using ambulatory monitoring with acoustic cardiography in an asymptomatic population 9. However, in chronic and acute heart failure patients using ambulatory acoustic cardiography Dillier et al. found much higher prevalence of percentage of S3 (chronic HF 13.0 ± 20.0%; acute HF 25.0 ± 22.0%) 10. Thus, our findings confirm those of Dillier et al. in an asymptomatic population and suggest a high percentage of S3 detected at night has good specificity as a marker of cardiac disease.
The fourth heart sound occurs in the active filling phase of diastole during atrial contraction by the abrupt deceleration of the A wave into a noncompliant left ventricle. Previously, the S4 has been associated with cardiac diseases that cause diastolic stiffness such as aortic stenosis, ischemia and left ventricular hypertrophy 23, and with worse prognosis following myocardial infarction 24. Detected by auscultation or phonocardiography in healthy persons, the prevalence of the S4 was as high as 73% and considered, by some, to be a normal result of aging 25, 26. However, more recent studies with acoustic cardiography have shown the presence of an S4 to be associated with LV stiffness 27. With aging, we found an increasing percentage of S4 detected during sleep in agreement with Dillier et al. using ambulatory acoustic cardiography in asymptomatic adults 9 suggesting changes in LV diastolic properties with increasing age.
Prolonged electromechanical activation time is related to the acceleration of pressure in the left ventricle and thus, a marker for LV systolic dysfunction. Roos et al. studied a population of 108 patients undergoing cardiac catheterization 7 with simultaneous acoustic cardiography. In the group with LV systolic dysfunction (defined as maximum LV dP/dt < 1600 mmHg / sec) they found EMAT to be prolonged (106 ± 21 vs. 85 ± 11 msec), and the diagnostic performance of EMAT superior to QRS duration and angiographic ejection fraction. We report a mean EMAT of 94.2 ± 12.4 ms during sleep which is consistent with the thresholds published previously for a population without overt cardiac disease. Further, there are no significant age-related increases of EMAT in our population consistent with the findings of Dillier et al. in an asymptomatic population 9 and in contrast to the increased night-time EMAT values seen by Dillier et al. in a heart failure population 10.
Efstratiadis and Michaels studied EMATc (EMAT divided by the cardiac cycle interval) in a population undergoing contemporaneous acoustic cardiography, cardiac catheterization and echocardiography 28. They defined abnormal EMATc as ≥ 15% and found it to be strongly associated with impaired LV contractility but not associated with elevated LV filling pressure. Sung et al. used EMATc at a threshold of 15% to guide outpatient therapy of patients with acute heart failure 29 in a prospective single-blind randomized study. One arm was conventional, symptom-guided (n=92) without the knowledge of EMATc while the other EMATc guided group (n=102) had a goal to reduce EMATc to less than 15%. Mean follow-up was 142 days and the EMATc-guided management group was superior to the symptom-guided group with significantly better clinical outcomes (27 events vs 39 events). We present the first results of percentage of EMATc ≥ 15% during sleep from ambulatory acoustic cardiography in an asymptomatic population and report a very low median of 0.2% consistent with a non-acute cohort.
We also report the first results for percentage of SDI ≥ 5.0 and SDI ≥ 7.5 during sleep using ambulatory acoustic cardiography in a non-acute population. Using short acoustic cardiography recordings, SDI ≥ 5.0 has been shown to indicate LV systolic dysfunction and SDI ≥ 7.5 to indicate severe LV systolic dysfunction with elevated filling pressures using independent correlates of echocardiography ejection fraction and cardiac catheterization for LV end-diastolic pressure 30. We observed very low percentages of SDI above threshold detected across all age groups suggesting high specificity of detection of acute cardiac disease.
We found a trend for higher likelihood of SDB for males than for females across all age groups, a significant increase in the likelihood of SDB for females with increasing age and an increasing trend of nocturnal non-dipping of heart rate with aging in males. Using acoustic cardiography in an asymptomatic population, Dillier et al. found a prevalence of 19% of SDB and 9.5% rate of nocturnal non-dipping of heart rate 11 whereas we found 17.6% of our population had high likelihood of SDB and 22% of those had nocturnal non-dipping of heart rate. These are important findings as both sleep disordered breathing and heart rate, particularly non-dipping of heart rate during sleep, to be predictors of cardiovascular and non-cardiovascular risk. Eguchi et al. followed 457 patients being treated or evaluated for hypertension 14 for an average of 72 months and found the risk of future cardiovascular events (stroke, myocardial infarction or sudden cardiac death) or mortality was 2.4 times greater in the patients without the typical night-time heart rate decrease. In a cohort of 3,957 patients treated for hypertension, Ben-Dov et al. assessed the determinants and mortality associations of sleep heart rate and noctural non-dipping 31 and found nocturnal non-dipping was independently associated with all-cause mortality. The relationship between nocturnal non-dipping and sleep apnea was studied by Sasaki et al. in a group of 251 patients with obstructive sleep apnea syndrome 32 over a mean follow-up period of 43 months. The non-dipping heart rate group had significantly more cardiovascular events including stroke, heart failure and ischemic heart disease and the presence of non-dipping was independently associated with the incidence of CVD.
Limitations of this study include the small number of participants in this first data collection so that more complete subgroup analysis by age and gender is not possible. Additional statistical analyses after stratification by SDB or BMI are also not possible due to small subgroup sizes. Echocardiographic parameters were not collected during the patient assessment, thus not allowing acoustic cardiography parameters to be correlated to conventional measures of LV systolic and diastolic function obtained in the same patient. Acoustic cardiographic analysis is limited in two ways. First, the acoustic cardiography analysis depends upon the quality of the ECG signal such that poor ECG signal due to artifact or other noise may reduce the analysis yield in the same way it does in regular ECG Holter analyses. Second, acoustic cardiography measurements are not calculated for heart rates greater than 150 bpm, which has not been a problem in this study population.
Conclusion
Ambulatory monitoring with acoustic cardiography provides the means in a primary care setting to screen for sleep disordered breathing and provides markers of left ventricular systolic and diastolic dysfunction. The acoustic cardiography findings showed a low percentage of patients with abnormal systolic dysfunction and age-related increases in diastolic dysfunction in this non-acute population. The proportion of subjects with sleep disordered breathing increased with aging and was associated with known risk factors such as obesity. These findings represent the baseline characteristics of this population that will be re-evaluated longitudinally. This initial data collection for SWICOS showed that ambulatory monitoring with acoustic cardiography is a viable and informative technique.
Conflicts of interest
P. Bauer and P. Arand work for Inovise Medical, Inc. which produces the acoustic cardiography monitoring devices used in this study. Inovise Medical provided the analysis results for the acoustic cardiography recordings and were blinded to all clinical information of the subjects.
Acknowledgements
Inovise Medical, Inc. provided the equipment to perform the acoustic cardiography procedure. SHK provided funds to cover research assistance and other study related costs.
References
- 1.Erne P, Bolli P, Burgisser E, Buhler F R. (1984) Correlation of platelet calcium with blood pressure. Effect of antihypertensive therapy. , N Engl J Med 310(17), 1084-1088.
- 2.Radovanovic D, Erne P. (2010) AMIS Plus: Swiss registry of acute coronary syndrome. Heart. 96(12), 917-921.
- 3.Schoenenberger A W, Muggli F, Parati G, Gallino A, Ehret G.. Protocol of the Swiss Longitudinal Cohort Study (SWICOS) in rural Switzerland, BMJ Open,2016 .
- 4.Erne P. (2008) Beyond auscultation – acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss medical weekly 138(31-32):. 439-452.
- 5.Shah S J, Nakamura K, Marcus G M, Gerber I L, McKeown B H. (2008) Association of the fourth heart sound with increased left ventricular end-diastolic stiffness. , J Card Fail 14, 431-436.
- 6.Shah S J, Michaels A D. (2006) Hemodynamic correlates of the third heart sound and systolic time intervals. Congest Heart Failure.12(Suppl1):. 8-13.
- 7.Roos M, Toggweiler S, Jamshidi P, Zuber M, Kobza R. (2008) Non-invasive detection of left-ventricular systolic dysfunction by acoustic cardiography in cardiac failure patients. , J Card Fail 14(4), 310-319.
- 8.Dillier R, Baumann M, Young M, Erne S, Schwizer B. (2012) Continuous respiratory monitoring for sleep apnea screening by ambulatory hemodynamic monitor. , World J of Card 4(4), 121-127.
- 9.Dillier R, Zuber M, Arand P, Erne S, Erne P. (2010) Assessment of systolic and diastolic function in asymptomatic subjects using ambulatory monitoring with acoustic cardiography. Clin Cardiol. 34(6), 384-388.
- 10.Dillier R, Zuber M, Arand P, Erne S, Erne P. (2011) Assessment of systolic and diastolic function in heart failure using ambulatory monitoring with acoustic cardiography. Annals of Med. 43(5), 403-411.
- 11.Stringhini S, Spencer B, Marques-Vidal P, Waeber G, Vollenweider P.Age and gender differences in the social patterning of cardiovascular risk factor in Switzerland: the CoLaus study. , Plos ONE 7(11), 49443.
- 12.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D et al. (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 3(4), 310-318.
- 13.Sung S H, Yu W C, Cheng H M, Chang Y P, Chen C H.Use of acoustic cardiography to guide outpatient therapy of patients with acute heart failure syndrome. JACC. 2014; 63 (12) (supplement A):. 1186-182.
- 14.Eguchi K, Hoshide S, Ishikawa J, Pickering T G, Schwartz J E. (2009) Nocturnal Non-dipping of heart rate predicts cardiovascular events in hypertensive patients. , J Hypertens 27(11), 2265-2270.
- 15.Cappuccio F P, Cooper D, D’Elia L, Strazzullo P, Miller M A. (2011) Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. , Eur Heart J 32, 1484-1492.
- 16.Ford E S. (2014) Habitual sleep duration and predicted 10-year cardiovascular risk using the pooled cohort risk equations among US adults. , J Am Heart Assoc.3e001454
- 17.Lee D C, Johnson R A, Bingham J B. (1982) Heart failure in outpatients: a randomized trial of digoxin versus placebo. , N Engl JMed; 306(12), 699-705.
- 18.Drazner M H, Rame J F, Stevenson L W, Dries D I. (2001) Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. , N EnglJ Med; 345(8), 574-581.
- 19.Maisel A S, Gilpin E, Hoit B. (1985) Survival after hospital discharge in matched population with inferior or anterior myocardial infarction. , J Am Coll Cardiol 6(4), 731-736.
- 21.Marcus G M, Gerber I L, McKeown B H, Vessey J C, Jordan M V. (2005) Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. , JAMA 293, 2238-2244.
- 22.Collins S P, Lindsell C J, Peacock W F, Hedger V D, Askew J. (2006) The combined utility of an S3 heart sound and B-type natriuretic peptide levels in emergency department patients with dyspnea. , J Card Fail 12(4), 286-292.
- 23.Stefadouros M A, Little R C. (1980) The cause and clinical significance of diastolic heart sounds. , Arch Intern Med 140, 537-41.
- 24.Ishikawa M, Sakata K, Maki. (1997) Prognostic significance of a clearly audible fourth heart sound detected a month after an acute myocardial infarction. , Am J Cardiol 80(5), 619-621.
- 25.Spodick D H, Quarry V M. (1974) Prevalence of the fourth heart sound by phonocardiography in the absence of cardiac disease. , Am Heart J 87(1), 11-14.
- 26.Swistak M, Mushlin H, Spodick D H. (1974) Comparative prevalence of the fourth heart sound in hypertensive and matched normal persons. , Am J Cardiol 33(5), 614-616.
- 27.Shah S J, Nakamura K, Marcus G M, Gerber I L, McKeown B H. (2008) Association of the fourth heart sound with increased left ventricular end-diastolic stiffness. , J Card Fail 14, 431-436.
- 28.Efstratiadis S, Michaels A D. (2008) Computerized Acoustic Cardiographic Electromechanical Activation Time Correlates with Invasive and Echocardiographic Parameters of Left Ventricular Contractility. , J Card Fail 14(7), 577-582.
- 29.Sung S H, Yu W C, Cheng H M, Chang Y P, Chen C H.. Use of Acoustic Cardiography to Guide Outpatient Therapy of Patients with Acute Heart Failure Syndrome. JACC. 2014; 63 (12) (supplement A): 1186-182.
- 30.Shapiro M, Moyers B, Marcus G M, Gerber I L, McKeown B H. (2007) Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. , J Card Fail 13, 18-24.
Cited by (3)
- 1.Gorshkov Yu. G., Volkov A. K., Voinova N. A., Shchukin S. I., 2020, Acoustocardiography with Assessment of Emotional Tension from the Voice, Biomedical Engineering, 53(6), 383, 10.1007/s10527-020-09948-8
- 2.Zhang Fu Wei, Zhang Yi Xue, Si Liang Yi, Chen Mo Shui, Wang Wei Wei, et al, 2021, Value of acoustic cardiography in the clinical diagnosis of coronary heart disease, Clinical Cardiology, 44(10), 1386, 10.1002/clc.23694
- 3.Mohamed Nourelhuda, Kim Hyun-Seok, Mohamed Manal, Kang Kyu-Min, Kim Sung-Hoon, et al, 2023, Tablet-Based Wearable Patch Sensor Design for Continuous Cardiovascular System Monitoring in Postoperative Settings, Biosensors, 13(6), 615, 10.3390/bios13060615
