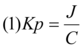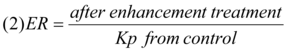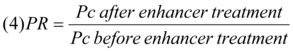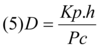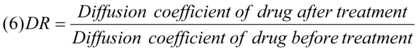Synthesis and Assessment of a New Tetrahydrogeraniol Derivative as Penetration Enhancer for Transdermal Drug Delivery
Abstract
Background:
Skin is one the most important sites for administration of drugs to obtain desired pharmacological effects either locally or through systemic bioavailability; and this has placed the transdermal route of drug delivery as an attractive and as one of the most innovative areas for conducting drug delivery research. However the stratum corneum in skin creates hurdles and acts as significant barrier for the permeation of drugs through skin. Penetration enhancers play a pivotal role to overcome such barriers and help enhance the permeation of drug through skin. However, penetration enhancement technology is challenging development and needs to be properly and skillfully addressed.
Objective:
The present investigation aimed to study the penetration enhancing effect of a newly synthesized alcohol derivative of an acyclic monoterpene (Tetrahydrogeraniol-THG).
Methodology:
The new derivative, 5,9-Dimethyl-1-Decanol (DIMDOL), has been synthesized by a chemical reaction of the THG with Grignard reagent and ethylene oxide. Permeation enhancing effect of the synthesized derivative was explored for better transdermal penetration of the two model drugs viz. tramadol hydrochloride and 5-fluorouracil (5-FU) through the excised rat skin by conducting in-vitro permeation experiments employing Franz diffusion cells apparatus. The standard enhancers Azone and THG were used to compare penetration enhancing effect of the enhancers.
Results:
It was revealed that DIMDOL could effectively enhance the permeability of both the drugs by 18.60 and 73.19 folds across the skin used with a lag time of 3.35 and 1.20 h, respectively. The newly synthesized derivative was found to significantly increase the partition coefficient and diffusion coefficient values.
Conclusion:
The results obtained suggest that DIMDOL can more effectively enhance the permeation of these model drugs, expectedly by affecting the stratum corneum and interacting with both lipid-rich layers and keratin-rich layers of the excised rat skin.
Author Contributions
Academic Editor: Kentaro Kato, Department of Parasitology, Institute of Tropical Medicine, Nagasaki University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Amjad Khan, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Transdermal drug delivery is the delivery of drugs across epidermis to achieve systemic effects. It is a non-invasive method for drug administration with an improved approach and is capable of maintaining therapeutic plasma drug level for prolonged and extended period of times 1. Properly designed and developed transdermal drug delivery systems (TDDS) may offer better solutions to the problems associated with other drug delivery systems currently in vogue. The systems have thus been developed as an alternative to oral and parenteral pharmaceutical forms. However, many drugs are unsuitable for use as transdermal therapeutic systems because of their low permeability through the human skin. The improvement of permeability using penetration enhancers may therefore be desirable 2. During the last few years various penetration enhancers including aprotic solvents, several surfactants and Azone have been extensively studied by different investigators in order to explore their penetration enhancing effect. The exact mechanism of penetration enhancing effect of these enhancers over the skin is not very clear, however, the lipophilicity of such enhancers is considered to play an important role in this regard. It has been found that mostly the long-chain primary alcohols could be of greater value in this connection, apart from being cheaper and alos non-toxic in nature 3 and the number of carbon atoms in the chain may influence the enhancing effect.
In our series of research work, previously we reported the synthesis of 5,9-Dimethyl-2-Decanol (DICNOL) and its evaluation as a potential percutaneous penetration enhancer4. The purpose of the present work is to synthesize 5,9-Dimethyl-1-Decanol (DIMDOL), another new derivative of Tetrahydrogeriniol (THG) and to investigate its enhancing effects on the penetration and transport of 5-Fluorouracil and Tramadol HCI through excised rat skin.
Materials and Methods
Apparatus
IR spectra were obtained with Perkin Elmer-983 spectrometer. NMR spectra were recorded on PMX-60si instrument. Mass spectra were obtained with ZAB-HS-VG analytical organic mass spectrometry. Gas chromatography was performed with Shimadzu gas chromatographer GC-9A with C-R3A chromatopac. Permeation studies were performed employing Franz diffusion cells (PermeGear, Bethlehem, PA).
Materials
Azone (Guangzhou Zhuji Factory, China), 5-Fluorouracil (Shanghai 12th Pharmaceutical Manufacturing Factory, China) and tramadol hydrochloride (Shi Jia Zhuang No. 1 Pharmaceutical Factory, China) were purchased locally. Tetrahydrogeraniol (Nanjing Perfume Factory, China) was also purchased from the local market and was purified up to contents of 99.9%. The remaining reagents used were purchased from the local market and all of them were analytical grade. The male white rats (Sprague Dawley) were obtained from the Animal House of the Zoology Department, University of Peshawar, KPK, Pakistan.
Preparation of Tetrahydrogeranyl Chloride (C10 H21 CI)
Tetrahydrogeranyl Chloride was prepared in the way as described in our previous reports 4, 5. In brief, 56 ml (47.04g, 0.297 mol) tetrahydrogeranyl and 150 ml dry acetonitrile were mixed with 100 g (0.38 mol) triphenylphosphine. This mixture was shaken at 25 0C, followed by slow addition of 43 ml (68 g, 0.441 mol) dry carbon tetrachloride into it. The mixture was at 25 0C for 6 h and then left overnight. The supernatant solvent was removed under reduced pressure and the oily precipitate was filtered off with petroleum ether through a column containing dried silica gel. Petroleum ether was evaporated and the oil obtained was distilled to give tetrahydrogeranyl chloride; yield: 42 g (80 %) percentage purity (97.10 %) was fond by gas chromatography.
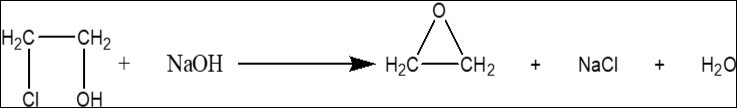
Preparation of Ethylene Oxide
In a three-necked flask, 50 ml of 2-ethylene chlorohydrins (95%) was taken, heated while stirring, followed by drop-wise addition of 45 ml of 40 % sodium hydroxide solution at 45 0C. A white precipitate was formed by the reaction of sodium hydroxide solution. The temperature of the reaction mixture began to fall and reached to 35 0C within 45 minutes. When the whole solution of sodium hydroxide was dropped into the flask, then the mixture was heated to about 90 0C and collected the ethylene oxide gas into a tube placed in ice-salt bath 6.
Synthesis of 5,9-Dimethyl-1-Decanol (C12 H26 O)
The 5,9-dimethyl-1-decanol (DIMDOL) was synthesized by preparation of Grignard reagent 7. For this purpose, 1.5 g of magnesium turnings, 4 ml of anhydrous ether and a crystal of iodine were taken in a four-necked flask, fitted with a condenser and 48 ml of anhydrous ether was slowly dropped into the reaction mixture. A gentle reflux was maintained with the aid of a lamp until most of the magnesium had reacted. The stirring was stopped and the reaction mixture flask was cool in a freezing mixture of ice and salt. Removed the dropping funnel and replaced it by a tube, the end of which was about 2 cm above the surface of the liquid. This delivery tube was attached to a flask containing ethylene oxide. Gradually introduced 4 ml of ethylene oxide into the reaction flask over a period of 1 h and kept the temperature below 10 0C. When the required ethylene oxide has been added, removed the freezing mixture surrounding the four-necked flask and kept the stirring continued. Temperature of the mixture gradually rose; at this time the mixture had become so viscous that stirring was no longer effective. Stopped the stirring, allowed to cool and decomposed the reaction mixture with saturated solution of ammonium chloride for hydrolysis. Separated the upper oily layer and evaporated the ether from the oily product using a rotary evaporator. The oily product was purified in a column by using dried silica gel and petroleum ether. It was distilled to give 7 g (63 %) of 5,9-dimethyl-1-decanol. Percentage purity was 99.55 % IR (neat) : γ 3350, 1060, 2950, 1465, 1380, 1370 cm-1; H1- NMR (CDCI3) δ (PPM) 0.83-0.89 (9h,d,j=6.1Hz, 3CH3), 1.00-1.75 ((14H,m,6CH2,2CH), 1.41 (1H, S, OH, D2O disappearing on deuterium exchange), 3.64 (2H, t, J=6.5 Hz, CH2OH); MS m/e no M+ , 168 (M-18, 11.2 %), 83, 69, 57).
Preparation of Skin
For this purpose, male white rats (Sprague Dawley) weighing 200 - 250 g were used. The animals were sacrificed and hairs from the abdominal side were cleaned off carefully. After it, a rectangular shaped section of the cleaned skin was excised and the fat and other visceral debris were removed from the under surface with care using tweezers. The excised skin was placed in normal saline for 12-16 hrs prior to its use for tissue hydration. It is worth mentioning here that the Ethical Review Committee of Quaid-i-Azam University had granted formal approval for all the protocols of this study.
Drug Permeation Studies
Permeation studies were conducted using an established procedure 4, 8. In brief, the experiments were performed by mounting fully hydrated skin samples in Franz diffusion cells (PermeGear, Bethlehem, PA), exhibiting a surface area of 0.64 cm2 available for diffusion with a receptor compartment volume of 5.3 ml. The receptor cells of the Franz Diffusion Cell apparatus were loaded with isotonic normal saline solution that had already been degassed by sonication for 5 min (Camlab Transsonic T310, Cambridge, UK). The isotonic normal saline solution in the receptor cells were maintained at 37 ± 0.5 °C. For this purpose a thermostatic water pump (Haake DC10, Karlsruhe, Germany) was used that could circulate the warm water through the chamber jacket of each cell while stirring the same continuously at 600 rpm. In order to facilitate hydration of the membranes, they were initially retained for 12 -16 hrs in the Franz cells, followed by inoculation of 1 ml saturated aqueous donor solution of either 5-FU or tramadol HCI on to each membrane surface. In order to minimize evaporation, each donor compartment was covered with a tight layer of Parafilm. After predetermined time intervals, 3 ml of the receptor fluid was withdrawn for analysis and immediately replaced with the same volume of warm medium to keep the volume constant. The concentrations values for the two drugs were determined spectrophotometrically at wavelengths of 266 nm and 271 nm for 5-FU and tramadol hydrochloride, respectively. All the experiments were carried out for 24 hours.
Scheme 3.Synthesis of 5,9-dimethyl-1-decanol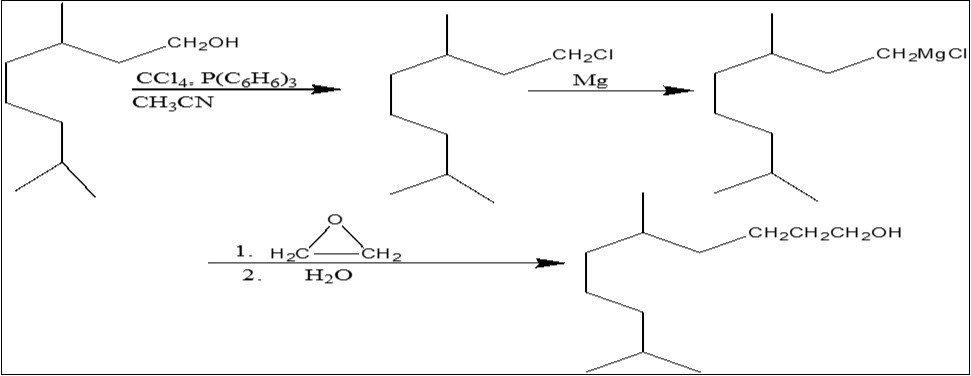
To determine the comparative enhancing effect of DIMDOL, THG and Azone the experiments were carried out in the same way but here the donor cell received 0.15 ml of DIMDOL, THG or Azone. After 12 h treatment, the excessive enhancer was removed by swabbing with tissue paper and replaced with 1 ml of saturated solution of 5-FU or tramadol hydrochloride. At predetermined time intervals, about 3 ml of the receptor medium was taken out for analysis and immediately replaced with 3 ml medium.
Determination of Amount of 5-FU and Tramadol Hydrochloride in skin
For this purpose the exposed skin was removed after the experiment was over. In order to remove excess drug on the surface, it was carefully washed with water for several time followed by dry blotting, weighing and cutting into many small pieces. The tissue was processed with 4 ml of ethyl acetate for three times in a homogenizer. The homogenates so obtained were mixed, vacuum filtered and ethyl acetate evaporated to dryness. The resultant residue was then reconstituted with 10 ml of water and analyzed for drug contents spectrophotometrically 9.
Data Analysis for Estimation of Permeation Parameters
Using the linear regression analysis method 10, the flux (µg / cm2.h) and lag time of 5-FU and tramadol HCI released from each system were calculated from the slope and the intercept of the plot obtained from the cumulative amounts of the drugs in the receptor fluid at steady state against time. Accordingly, the flux would be equal to the slope and lag time to the x-intercept of the linear portion of the curve. The following equation 8 may be used to calculate the permeability coefficient (Kp) of the drugs.
In equation (1), ‘J’ denotes the flux and ‘C’ the concentrations of the drugs added to the skin. Evaluation of permeability coefficients (Kp) of skin to the drugs prior and after treatment with penetration enhancers may permit the activity of the enhancers to be expressed in the form of enhancement ratio (ER) 11 in the following way:
The apparent partition coefficients (PC) of the drugs are expressed by the following equation 12.
The partitioning of the drugs into the skin is illustrated by the partition ratio (PR) 13 as follows:
The apparent diffusion coefficients (D) of 5-FU and tramadol HCI were calculated, using the following equation13.
Where Kp is the mean permeability coefficients, Pc is the apparent partition coefficient and h is the thickness of the stratum corneum (taken as 3 X 10-3 cm). The increase in the diffusivity of drugs in the skin may be expressed as diffusivity ratio (DR) 13.
Results
DIMDOL was synthesized in two steps. In the first step, THG was reacted with acetonitrile, triphenylphosphine amd carbpm tetahydrogeranyl chloride. The reaction described illustrates a general procedure for the preparation of allylic chlorides from allylic alcohols without rearrangement and under conditions allowing the retention of sensitive groups. In the second step, tetrahydrogeranyl chloride was treated with Grignard reagent and ethylene oxide to give DIMDOL. The chemical structure was verified by IR, NMR and Mass Spectra.
Effect of DIMDOL on in Vitro Penetration of 5-FU
The permeation parameter values: Mean fluxes (J), permeability coefficients (Kp) and enhancement ratios (ER) of 5-FU before and after treatment of skin with DIMDOL, THG and Azone are shown in Table 1. The permeation profiles of the mean quantity permeated for 5-FU accumulated against time in presence and absence of enhancers are shown in Figure 1.
Table 1. Mean fluxes (J), permeability coefficients (Kp) and enhancement ratios (ER) of 5-FU in excised rat skin before and after treatment with DIMDOL, THG and Azone (n = 5~ 10, mean ± S).| Enhancers | Flux (µg/cm2) | Kp (cm/h X 10-3) | ER |
| Control | 18.13± 1.35 | 1.45±0.10 | ……. |
| DIMDOL | 336.97±23.14 | 26.96±1.85 | 18.60±1.27 |
| THG | 875.14±70.14 | 70.01 ± 5.60 | 48.30 ±3.87 |
| Azone | 1498.82 ±90.09 | 119.91±7.21 | 82.72 ± 4.97 |
Figure 1.Penetration profiles of 5-FU in the presence of DIMDOL, THG and Azone. Each point shows the mean ± SD of 5 to 10 skin samples.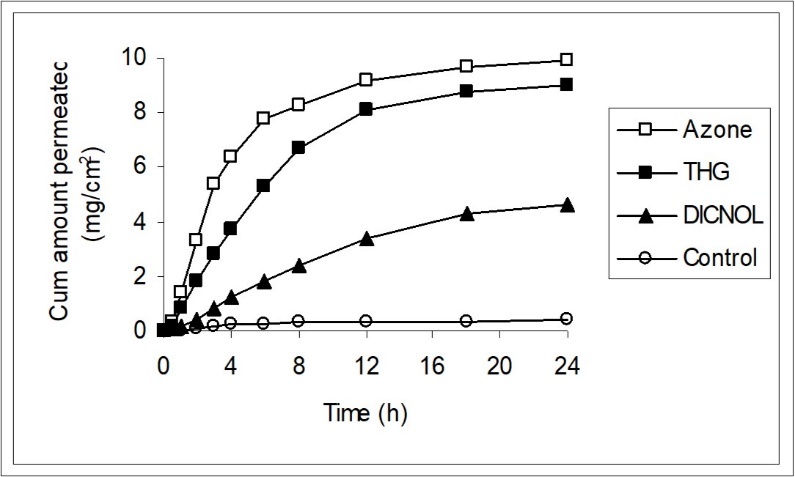
The mean control value of J and Kp for 5-FU in the untreated skin is 18.13±1.35µg/cm2/h and 1.45 ± 0.10 X 10-3cm/h with a lag time of 4hr, respectively. The steady state permeation was observed for 12hr when DIMDOL was used as enhancer, while with THG and Azone, the steady state conditions were observed for 6 and 4h, respectively. DIMDOL increased the penetration of 5-FU about 19 fold with a lag time of 3.5hr.The partitioning results with these enhancers are shown in Table 2. Treatment of the skin with DIMDOL increased the partitioning of the drug into the skin as the mean untreated PC value of 5-FU is 34.43 ± 4.70 X 10-3 only.
From Table 2 it can be seen that all the three enhancers has decreased the resistance to the diffusion of 5-FU as the mean untreated apparent diffusion coefficient value is 0.135±0.02 X 10-3/hr only.
Table 2. Mean apparent partition coefficients (PC), apparent diffusion coefficients (D), partition Effect of DIMDOL on in vitro penetration of tramadol HCI ratios (PR) and diffusion ratios (DR) of 5-FU into fully hydrated skin (n=5~10, mean ± S).| Enhancers | PC | D | PR | DR |
| X102 | cm/h X 10-3 | |||
| Control | 34.43± 4.70 | 0.135±0.02 | …. | …. |
| DIMDOL | 129.56±11.14 | 0.63±0.08 | 3.08 | 4.7 |
| THG | 65.36±4.28 | 2.89 ± 0.55 | 1.9 | 21.5 |
| Azone | 92.03 ±6.54 | 3.93±0.47 | 2.7 | 29.21 |
Figure 2 shows a typical time-course profile of tramadol HCI for DIMDOL, THG and Azone. Each data point represents of the mean value from 3 - 6 trials. Calculated fluxes (J), permeability coefficients (Kp) and relative enhancement ratios (ER) are shown in Table 3. The control value for J and Kp of of tramadol HCI in the untreated skin at 37± 0.50C is 60.41 ± 9.29µg/cm2/hr and 0.20 ± 0.03 cm/hr X 10-3 with a lag time of 6hr, respectively.
Table 3. Estimated mean fluxes (J), permeability coefficients (Kp) and enhancement ratios (ER) for tramadol HCI in the absence and presence of enhancers) n= 3~6, mean± S).| Enhancers | Flux (µg/cm2 /h) | Kp (cm/h x 10-3) | ER |
| Control | 60.41 ± 8.29 | 0.20 ± 0.03 | ……… |
| DIMDOL | 7793.81± 603.70 | 15.59±1.21 | 77.94±6.04 |
| THG | 11133.21±1101.93 | 22.27 ± 2.20 | 109.69 ±10.86 |
| Azone | 9449.33±1296.17 | 18.90±2.59 | 93.04 ± 12.88 |
Figure 2.The penetration profiles of tramadol HCI in the presence of DIMDOL, THG and Azone. Each point shows the mean ± SD of 3 to 6 skin samples.
When the skin was treated with DIMDOL, the lag time for tramadol HCI falls and was observed to be 1.5hr, while with THG and Azone it was observed to be 10 - 15 minutes.
The steady state permeations were observed only for 6hr with these enhancers, which may contribute to accumulation of higher concentrations of tramadol HCI in the receptor cell and /or wash up effect of the enhancers in diffusion cells. DIMDOL caused a 78-fold increase in tramadol HCI permeation across the exercised rat skin, while THG and Azone increased the drug permeation to 110 and 93-fold, respectively. The partitioning results with these enhancers are shown in Table 4.
Form Table 4 it can be seen that all the three enhancers has decreased the resistance to the diffusion of tramadol HCI as the mean untreated diffusion coefficient value of the drug is 0.11± 0.02 X10-3 cm2 /hr only, while with DIMDOL this value was observed to be 2.64 ± 0.13 X 10-3 cm2/hr.
Table 4. Estimated mean apparent partition coefficients (PC), apparent diffusion coefficients (D), partition ratios (PR) and diffusion ratios (DR) of tramadol HCI into fully hydrated skin (n=3~6, mean ± S).| Enhancers | PC X102 | D (cm/h X 10-3) | PR | DR |
| Control | 5.77± 0.42 | 0.11 ± 0.02 | ……… | …….. |
| DIMDOL | 17.73±11.14 | 2.64 ± 0.13 | 3.07 | 24.91 |
| THG | 47.50± 3.37 | 1.41 ± 0.11 | 8.23 | 13.27 |
| Azone | 2 1.45±3.36 | 2.67±0.42 | 3.72 | 25.17 |
Discussion
Penetration enhancers are generally reported as substances which could temporarily reduce the barrier action of the stratum corneum (SC) 14. Stratum corneum is the major barrier to transdermal transport of drugs and the mechanism of drug transport through this barrier is considered to be through the process of partitioning and diffusion that may be directly influenced by the molecular properties of the penetrants including the solubility, size and shape 15. Transdermal drug absorption involves the processes of drug partitioning from the vehicle into the stratum corneum, diffusion of drug through the stratum corneum, drug partitioning from stratum corneum to aqueous viable tissue, and drug diffusion through the viable tissue to the dermal microcirculation. If a penetration enhancer acts on any one or more of the first three processes described above, it is likely to be effective 16.
In the present work, we report the synthesis and enhancing effect of DIMDOL on the permeation of 5-FU and tramadol HCI using excised rat skin. DIMDOL is a THG derivative and was synthesized by the addition of two alkyl chain (CH2 –CH2) groups on the functuional moiety of the hydroxyl group of THG. It causes 19-fold increase of 5-FU penetration with a lag time of 3.5 hr, while it enhances the transdermal penetration of tramadol HCI about 78-fold with a lag time of 1.5hr, which was probably due to the slow penetration of DIMDOL into the skin. THG and Azone were used for comparison and showed 48 and 83-fold increase of 5-FU penetration, respectively. In case of tramadol HCI, THG and Azone caused 110 and 93-fold increase of drug penetration with a lag time of 10~15 minutes. The partition ratios calculated suggest that DIMDOL increase partition of 5-FU and tramadol HCI into the skin. As both drugs are less soluble in DIMDOL than in water this increased partition of drugs may be due to the retention of the drugs by skin, as in this case full thickness skin was used to determine drug contents. Therefore, we conclude that the enhanced permeation of 5-FUand tramadol HCI may not be only by increasing the partition of drugs into the SC but also by disrupting the lipid structure, thereby increasing the diffusion coefficient of the drugs in the membranes as observed from the diffusivity ratios being 4.70 and 24.91 for 5-FU and tramadol HCI, respectively (Table 2 & Table 4). Both drugs possess different dissociating properties and solubilities in water. As observed from the results, for 5-FU penetration the diffusion coefficient (D) is more important than partition coefficient (PC), while in case of tramadol HCI PC is more important than D, because, PC values were varied in the skin for DIMDOL and THG. It may conclude that the enhancers do not directly affect the skin but it might have resulted from the solubility of the both drugs in the enhancers. The results indicated that the enhancing efficiency does not only depend on the property of enhancer but also depends on the coordination between the drug and enhancer. The hydrophobic chain length is considered to be the cause for a greater apparent change in the overall activity of these compounds as penetration enhancers. For maximum penetration enhancing activity to be achieved, a different polar group may require a different hydrophobic group 17.
In light of the results obtained from this investigative study, it may be concluded that DIMDOL, our newly synthesized derivative containing terpene moieties from natural products possesses tremendous potential to enhance the transdermal penetration of the model drugs viz. 5-FU and tramadol HCI. Nevertheless, further focused studies are required in order to explore where in the skin these enhancers are functioning and in what way they are altering the permeability of the skin for the tested drug. For this purpose, more investigative studies are being carried out by our research group to explore the exact mechanisms of action of these compounds as better penetration enhancers.
Disclosure of Conflict of Interest
The authors of the research article do not have any type of financial and/or non-financial competing interests with other people or organization.
Authors Contributions
AK, GMK and MHR conceived of, designed and performed experimental work the study. GMK, MHR and AK, jointly worked on statistical analysis, preparation of the graphs and final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank the Department of Pharmacy, Quaid-i-Azam University, Islamabad for providing research facilities.
References
- 1.Panchagnula R, Bokalial R, Sharma P, Khandavilli S. (2005) Transdermal delivery of naloxone: skin permeation, pharmacokinetic, irritancy and stability studies. , Int. J. Pharm 293, 213-223.
- 2.William A C, Barry B W. (1989) Essential oils as novel human skin penetration enhancers. , Int J Pharm 57, 87-89.
- 3.Sinha V R, Kaur M P. (2000) Permeation Enhancers for Transdermal Drug Delivery. Drug Dev Ind Pharm. 26(11), 1131-1140.
- 4.Khan G M, Hussain A, Hanif R M. (2011) Preparation and Evaluation of 5, 9-Dimethyl-2-Cyclopropyl-2-Decanol as a Penetration Enhancer for Drugs Through Rat Skin. , Pak. J. Pharm. Sci 24(4), 451-457.
- 5.Hanif R, Ping Q N, Mo F Z, Gao Z. (1998) Enhancing effect of 4,8-Dimethyl-1-Nonanol on the transepidermal delivery of 5-fluorouracil and tramadol hydrochloride. , J. China Pharm. Univ 29(3), 170-175.
- 6.Zhang S G. (1992) . , Manual of Fine Organic Chemical Product Technology, Science Publishing House 2, 989-990.
- 7.Brain S F, Antony J H, Peter WGS, Austin R T. (1989) . Vogel’s Text Book of Practical Organic Chemistry, 5th ed. p536 .
- 8.Khan G M, Frum Y, Sarheed O, Eccleston G M, Meidan V. (2005) Assessment of Drug Permeability Distributions in Two Different Models Skins. , Int. J. Pharm 303, 81-87.
- 9.Tenjarala S N, Allen R.Borazani A (1994).Evaluation of verapamil hydrochloride penetration through human cadaver skin. Drug Dev Ind Pharm. 20(1), 49-63.
- 10.Julraht K, Keith A P, James W.A (1995).Development of a transdermal delivery devicefor melatonin in vitro study. Drug Dev Ind Pharm 21(12), 1377-1387.
- 11.William A C, Barry B W. (1989) Permeation, FTIR and DSC investigation of terpene penetration enhancers. in human skin.Pharm Res. 41: 12 P (Supplement)
- 12.Abdullah D, Ping Q N, Liu G J. (1996) Enhancing effect of eucalyptus oil and its β-Cyclodextrin complex on the penetration of 5-fluorouracil through excised rat skin. , J China Pharm Univ 27(2), 77-83.
- 13.William A C, Barry B W. (1991) Terpenes and lipid-protein-partitioning theory of skin penetration enhancement. Pharm Res. 8(1), 17-24.
- 14.Sharma S, Kulkarni J, Pawar A P. (2006) Permeation enhancers in the transmucosal delivery of macromolecules. , Pharmazie 61, 495-504.
- 15.Hashida M, Mukai E, Kimurat T, Sezaki H. (1985) Enhanced delivery for mitomycin-C derivatives through hairless mouse skin. , J Pharm Pharmacol 37, 542-544.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
G. Isopencu, C. Covaliu-Mierlă, I. Deleanu - Plants (2023) Semantic Scholar
Plants (2023) Crossref
Plants (2023) OpenAlex
Journal of Glycomics and Metabolism (2016) OpenAlex