Low Laser Therapy Plus Non-Surgical Periodontal Treatment Reduces Interleukin-1 Beta Serum Levels
Abstract
Objectives:
Periodontal disease is associated to widespread systemic inflammation, and further to both cardiovascular morbidity and mortality. Data from intervention studies demonstrated the beneficial effects of periodontal therapy in reducing vascular diseases. The present study was aimed to explore whether low-level Laser therapy as an adjunct to scaling and root planning reduces serum levels of inflammatory cytokines.
Material and Methods:
Thirty patients were enrolled. All recruited participants underwent blood sampling and dental inspection for periodontal indexes measurement. Plaque index, gingival index and probing depth were employed as measures of periodontal disease. Afterwards, patients underwent scaling and root planning plus low-level Laser therapy. Inflammatory biomarkers and periodontal indexes were measured before treatment and twenty weeks after treatment.
Results:
Plaque index, gingival index and probing depth largely improved at the follow-up visit, resulting more than halved from the baseline. Furthermore, a significant reduction of serum interleukin-1 beta has been observed (1.1 SD 2.1 vs 0.5 SD 1.3, P = 0.04), whereas serum interleukin-6 levels remained substantially unchanged. Blood C-reactive protein levels decreased at the follow-up, but not reaching statistical significance.
Conclusions:
therapy addressed to a local improvement of periodontal disease gives a reduction of systemic inflammation, possibly beneficial for cardiovascular health.
Author Contributions
Academic Editor: Rajiv Saini, associate Professor
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Maria Serena De Franceschi
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Periodontal disease is an inflammatory condition characterized by gingival inflammation and progressive destruction of the supporting tissues of the teeth.1 In the United States, it is reported a prevalence of about 47% in adults aged ≥ 30 years, whereas the 64% of adults aged ≥65 years have moderate/severe periodontitis.2 Periodontal disease is associated with widespread systemic inflammation, as demonstrated by the increase in circulating C reactive protein and inflammatory cytokines.3,4 Systemic inflammation is a potential contributor to atherosclerosis and/or thrombosis, pathologic bases of cardiovascular diseases.5 In fact, several studies indicate that plasma levels of inflammatory biomarkers, such as CRP, IL-6 or IL-1β, can be useful in identification of individuals at high risk for future cardiovascular events.6 Indeed, raised serum proinflammatory cytokines impair nitric oxide (NO) synthesis (an important vasodilator compound) and enhance endothelin-1 (ET-1) production.7, 8, 9This important topic is reinforced by evidences suggesting that non-surgical periodontal treatment should be able to restore the anti-atherogenic properties of the endothelium and to reduce serum cytokines levels.10,11
Interestingly, it seems that low levels Laser treatment (LLLT), as an adjunctive therapy to non-surgical periodontal treatment, improves periodontal healing and reduces interleukin burden locally, in the gingival crevicular fluid.12 It is not known if this improvement is also followed by a reduction of inflammation into the blood of systemic circulation.
The aim of the present investigation was to test the effect of periodontal therapy by Laser plus non-surgical periodontal treatment on serum levels of inflammatory cytokines in patients with periodontal disease.
Material and Methods
Patients and Study Design
The study was a prospective intervention trial with a twenty weeks follow-up. Participants were consecutively recruited among outpatients referred from their General Practitioners to the Dental Unit of "Magna Graecia” University in Catanzaro (Italy) for periodontal disease in the period between February and November 2013. Inclusion criteria were: age >18 and < 70 years, and periodontitis lesions needing full mouth therapy upon Dentist judgement. Exclusion criteria were: antibiotic therapy in the last three months, heart failure (class III or IV NYHA), active cancer, severe renal (stage II to V) and hepatic (Child-Pugh B-C) disease, and current autoimmune and infectious diseases.
The sample size was calculated considering a power of 0.80 (Zβ: 0.84), an alpha of 0.05 (Zα/2: 1.96) and an expected effect size of 0.52. A total of 29 subjects should be enrolled. Recruitment period was closed after enclosing the first 15 males and 15 females, therefore thirty eligible participants were recruited. All recruited subjects gave written informed consent. All participants underwent blood sample withdrawal, and complete clinical examination in a Metabolic Disease Unit of the same University. Medical history, pharmacological treatment(s) and smoking habit were recorded. All recruited patients were then scheduled for blood sampling and dental inspection for periodontal indexes measurement (baseline visit, V1). Afterwards, patients underwent full mouth therapy plus Laser therapy. The clinical follow-up was after twenty weeks of treatment (V2).
Staff members involved in laboratory procedures and dental inspections were blinded each other.
Biochemical and Clinical Parameters
Blood was withdrawn from an antecubital vein after 12 hour fasting. Blood lipids and fasting glucose were measured using commercially available kits. Participants having fasting glucose ≥ 126 mg/dl were invited to repeat blood withdrawal.
IL-1beta and IL-6 determinations were obtained by solid phase sandwich using enzyme linked immunosorbent assay ELISA (Diaclone SAS, Besancon, Cedex - France). For IL-1beta, the overall intraassay coefficient of variation has been reported to be 4.5%. High-sensitivity C-reactive protein (hs-CRP) was determined by a nephelometric system (Siemens BNII, Dade Behring, Marburg - German).
Clinical examination was held as previously described.13 Briefly, height and weight were detected using standard methods, and body mass index computed as weight (kg) divided by height squared (m2). Systolic (SBP) and diastolic blood pressure (DBP) was measured with a standardized sphygmomanometer on the right arm after the participant rested for at least 5 minutes. The average of the second and third of three readings was computed. Hypertension was defined as systolic and/or diastolic values respectively > 139 mmHg and > 89 mmHg, or current antihypertensive treatment. Diabetes was considered present if participant was on therapy, or if fasting blood glucose determination was ≥ 126 mg/dl in two different occasions. Hyperlipidemia was defined as total cholesterol ≥ 200 mg/dl and/or triglycerides ≥ 150 mg/dl, or on treatment with lipid lowering agents. Patients who smoked during the previous 12 months were classified as smokers.
Dental Evaluation
The degree of gingival inflammation was assessed by gingival index (GI), measured from 0 to 3 on each tooth: 0 = healthy gingiva; 1 = mild inflammation, absence of bleeding on probing; 2 = moderate inflammation, bleeding on probing; 3 = severe inflammation, spontaneous bleeding.14
The gingival plaque’s burden was assessed by plaque index (PI): 0 = absence of plaque; 1 = presence of plaque detectable with probe; 2 = moderate accumulation of plaque; 3 = abundant accumulation of plaque. GI and PI were detected on 6 teeth: 12, 16, 24, 32, 36, 44.15
Finally, probing depth (PD) index was used to have indications of deep inflammation involving also periodontal ligament, dental cementum and alveolar bone. A periodontal probe (PCP/15 AIR, Henry Schein, Milan, Italy), with millimetre markings 1 - 15, measured the distance from the gingival margin to the base of the sulcus or periodontal pocket along main axis of the tooth. The examiner calibrated his probing pressure between 10 and 20 grams before each patient examination.
The depth of dental pockets was detected at six sites in each tooth (distofacial, facial, mesiofacial, distolingual, lingual and mesiolingual).16 Probing depth was scored as: 0 to 2 mm = 0; 2.1 to 4 mm = 1; 4.1 to 6 mm = 2; > 6 mm = 3. A mean of all measurements for each tooth was considered. As known, periodontal indexes are usually a sum of mean scores by each dental element divided for the number of evaluated teeth, that is, a mean value for patient indicating the severity of the disease. As in a previous paper, here the sum value was also considered, indicating the spread of the disease in each patient.17
Periodontal Therapy
Patients underwent to a full mouth scaling and root planning treatment (SRP). After the initial treatment, patients received Laser therapy through a Laser device (Ezlase™ soft tissue diode Laser - Biolase Technology Inc. 4 Cromwell - Irvine, CA 92618), by 3 cycles of irradiation for each pocket, each lasting 30 seconds. This Laser therapy was the clinical standard for the Dental Unit in the study period; therefore, a control group was not constituted, being not ethical.
Statistical Analysis
Statistical analyses were performed by PASW 18.0 for Windows. Continuous variables are expressed as mean and standard deviation (M SD), whereas dichotomous variables as percentage. All studied variables had normal distribution, except IL-1β and IL-6. Student t-test for paired data or Wilcoxon test was used, as appropriate, to compare periodontal indexes and inflammatory markers at baseline and twenty weeks after therapy. Pearson correlation coefficient was used to verify the association between the percent variations at the follow up of the periodontal vs inflammation indexes. Statistical significance was set at p < 0.05.
Results
Thirty subjects, 15 females and 15 males, were recruited for the present study. Clinical and biochemical characteristics and prevalence of cardiovascular risk factors are reported in Table 1. Recruited patients were mainly middle aged people with a low cardiovascular risk profile. Regarding cardiovascular risk factors: 14 were hypertensive, 19 had hyperlipidemia and 3 had diabetes. Finally, 11 were currently smokers and 8 were obese. These clinical features were unchanged at the end of the study (data not shown).
Table 1. Baseline clinical, biochemical characteristics and cardiovascular risk factors of 30 recruited patients (15 females and 15 males)| Clinical parameters | Mean ( SD) |
| Age (years) | 55.2 (10.2) |
| Systolic blood pressure (mmHg) | 129 (14) |
| Diastolic blood pressure (mmHg) | 80 (8) |
| Fasting glucose (mg/dl) | 97.9 (8.9) |
| Total cholesterol (mg/dl) | 209 (47) |
| HDL cholesterol (mg/dl) | 50 (13) |
| Triglycerides (mg/dl) | 149 (144) |
| Cardiovascular risk factors | % |
|---|---|
| Smoking | 37 |
| Hypertension | 47 |
| Dyslipidemia | 63 |
| Obesity | 27 |
| Diabetes | 10 |
Table 2 reports periodontal indexes (expressed as mean and sum) and inflammatory markers of patients at the baseline and twenty weeks after therapy. As shown, periodontal indexes were largely improved at the follow-up visit, decreasing of 55 - 60% from the baseline (Figure 1).
Table 2. Periodontal indexes and inflammatory markers at baseline and twenty weeks after therapy| Periodontal indexes | Baseline Mean ( SD) | After twenty weeks Mean ( SD) | P |
| Plaque index (mean) | 1.9 (0.7) | 0.8 (0.5) | <0.001a |
| Gingival index (mean) | 1.8 (0.5) | 0.7 (0.6) | <0.001a |
| Probing depth (mean) | 1.8 (1.3) | 0.7 (0.8) | <0.001a |
| Plaque index (sum) | 43.9 (21.1) | 18.4 (12.1) | <0.001a |
| Gingival index (sum) | 41.7 (16.3) | 15.9 (13.6) | <0.001a |
| Probing depth (sum) | 41.1 (31.6) | 16.9 (17.2) | <0.001a |
| Inflammatory markers | |||
| Interleukin-1β (pg/mL) | 1.1 (2.1) | 0.5 (1.3) | 0.04b |
| Interleukin-6 (pg/mL) | 2.0 (1.7) | 2.2 (1.5) | N.s. |
| C-reactive protein (mg/L) | 5.5 (8.1) | 3.3 (3.2) | N.s. |
Figure 1.Periodontal indexes at baseline and twenty weeks after therapy. Data are expressed as mean (SD). *p< 0.001, Student t test for paired data.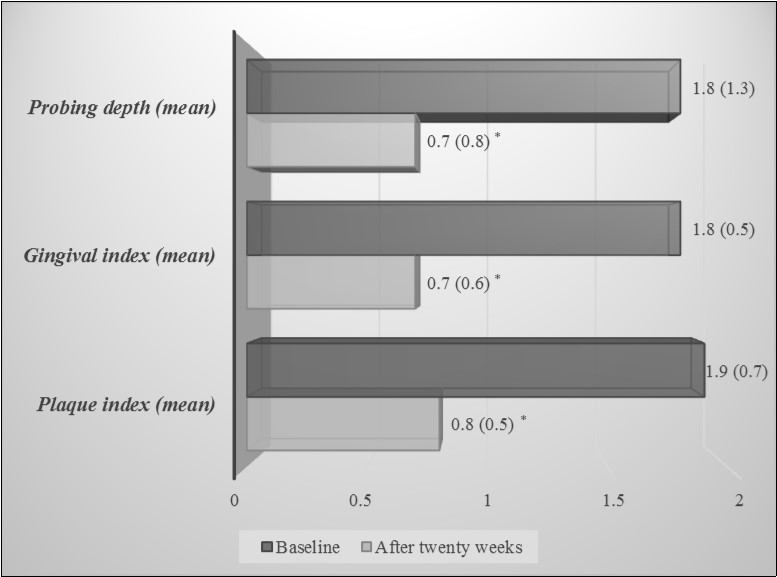
Regarding inflammatory markers, it has been observed a significant reduction of serum interleukin-1β twenty weeks after therapy (p = 0.04), whereas serum IL-6 levels remained substantially unchanged. Serum hs-CRP levels decrease at V2, but not reaching statistical significance (Figure 2).
Figure 2.Inflammatory markers at baseline and twenty weeks after therapy. Data are expressed as mean (SD). *p = 0.04, Wilcoxon test.
The percent variations at the follow up of the clinical periodontal indexes were not related to the percent variations of inflammation lab values (data not shown).
Discussion
The present investigation demonstrates the beneficial effects of a combined therapy, photodynamic plus nonsurgical treatment, in the improvement of periodontal indexes and in the reduction of serum inflammatory biomarkers, particularly interleukin 1β.
It is known that periodontal disease is not only a peripheral pathological condition but it is associated with several cardiovascular disorders such as endothelial dysfunction, atherosclerosis and major cardiovascular events.5 The mechanisms of this association may be found in the widespread systemic inflammation characterizing periodontal disease.3,4 On this basis, any treatment aimed to improve the oral health, it seems to restore the endothelium properties and reduce systemic inflammation.
In recent years the LLLT as an adjunct therapy has gained increased employment in common clinical practice and several studies have been published.18, 19, 20, 21, 22, 23 However, the results of these researches are often conflicting and various, probably due to the types of Laser and parameters of Laser radiation employed. In a case-control study, LLLT add on therapy improved not significantly oral health in terms of PD reduction, but it resulted in a significantly higher reduction in bleeding scores compared to non surgical treatment alone.24 Moreover, in a split-mouth study Lai et al.25 have analyzed the effect of low levels of He-Ne Laser treatment as an adjunct to SRP in sixteen patients with moderate to advanced chronic periodontitis. The authors failed to find any statistically significant difference in terms of clinical parameters or radiographic findings between the test and control sites.25
By contrast, another interventional study carried out by Makhlouf et al.26 demonstrated that combination therapy with 840 nm diode Laser improved clinical and radiographic findings after 6 and 12 months. However, did not significantly affect either the gingival crevicular fluid of IL-1β or the gingival or plaque index.26 Again, a short term clinical trial suggests that a combined course of photodynamic therapy with low-level Laser could be a beneficial adjunct to nonsurgical treatment of chronic periodontitis at 1 week and 1 month, but not at 3 months.27 Other authors demonstrated a beneficial effect of LLLT in selected populations only. Indeed, Dukić et al.28 indicates that, compared to SRP alone, multiple adjunctive applications of a 980-nm diode Laser with SRP showed PD improvements only in moderate periodontal pockets. Finally, LLLT as an adjunct in periodontal therapy has been demonstrated to reduce gingival inflammation in a large interventional trial involving three hundred patients with diabetes mellitus.29
Overall, the results of these interventional studies suggest that low level Laser irradiation can be used as a helpful adjuvant method of treatment, which, together with traditional periodontal therapy, leads to better and longer-lasting therapeutic results.
At the present, limited data are available on the potential effects of LLLT plus non-surgical periodontal therapy in reducing levels of systemic inflammation. The present investigation explores this important aspect in a follow-up period of twenty weeks. Our findings suggest that PI, GI and PD significantly improve after the combined treatment. Regarding inflammatory biomarkers, only serum IL-1β significantly decreases after combined therapy, whereas hs-CRP reduction not reached the statistical significance. Finally, no effects on serum IL-6 levels have been found.
IL-1β and IL-6 are cytokines with a broad range of humoral and cellular immune effects related to inflammation. The blockage of IL-1 activity results in a rapid and sustained reduction in disease severity, in a number of autoimmune and cardiovascular disorders.30, 31 Current data indicate that plasma levels of inflammatory biomarkers, can be clinically useful in the identification of individuals at high risk for future cardiovascular events.6 Indeed, these cytokines could affect the mechanism of vascular tone and the signaling pathways of vasoconstriction and vasodilatation, vascular cell growth and proliferation, and could also lead to structural changes in the vessel wall.7, 8, 9 In this scenario, LLLT plus non-surgical periodontal therapy might be useful not only for the improvement of oral health, but also to prevent endothelial dysfunction and atherosclerosis. Very recently, it has been also verified that LLLT elevates carotid wall shear stress in the medium term, determining an important change in physical arterial conditions protecting against cardiovascular diseases.32
The present study has, in our opinion, some limitations. First, the small sample size. The relatively low number of participants is in part justified by the complexity of examination. Second, the study design makes not possible to discriminate the effects of the two different treatments, SRP and LLLT.
Conclusions
In conclusion, the present investigation demonstrates an improvement of periodontal disease and a concomitant reduction of some blood inflammatory markers, in patients undergoing a periodontal therapy based on Laser plus conventional therapy. This effect might be a way by which the management of periodontal disease is beneficial for a reduction of cardiovascular diseases.
Acknowledgments and Disclosure Statements
The authors acknowledges Dr Claudio Carallo e Dr Cesare Tripolino for their help in the development of the study.
The authors report no conflicts of interest related to this study.
References
- 2.Thornton-Evans G, Eke P, Wei L, Palmer A, Moeti R et al. (2013) . Centers for Disease Control and Prevention (CDC). Periodontitis among adults aged ≥30 years-United States,2009-2010. MMWR Surveill Summ;62 Suppl3: 129-135.
- 3.Eberhard J, Grote K, Luchtefeld M, Heuer W, Schuett H et al. (2013) Experimental gingivitis induces systemic inflammatory markers in young healthy individuals: a single-subject interventional study. , PLoS One; 8, 55265.
- 4.Moutsopoulos N M, Madianos P N. (2006) Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. , Ann NY Acad Sci; 1088, 251-264.
- 5.Libby P, Ridker P M, Maseri A. (2002) Inflammation and atherosclerosis. , Circulation; 105, 1135-1143.
- 6.Blake G J, Ridker P M. (2002) Inflammatory bio-markers and cardiovascular risk prediction. , J Intern Med; 252, 283-94.
- 7.Khan D A, Ansari W M, Khan F A. (2011) Pro/anti-inflammatory cytokines in the pathogenesis of premature coronary artery disease. , J Interferon Cytokine Res; 31, 561-567.
- 8.Sprague A H, Khalil R A. (2009) Inflammatory cytokines in vascular dysfunction and vascular disease. , Biochem Pharmacol; 78, 539-52.
- 9.Vila E, Salaices M. (2005) Cytokines and vascular reactivity in resistance arteries. , Am J Physiol Heart Circ Physiol; 288, 1016-1021.
- 10.Seinost G, Wimmer G, Skerget M, Thaller E, Brodmann M et al. (2005) Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. , Am Heart J 149, 1050-1054.
- 11.Tonetti M S, D'Aiuto F, Nibali L, Donald A, Storry C et al. (2007) Treatment of periodontitis and endothelial function. , N Engl J Med; 356, 911-920.
- 12.Aykol G, Baser U, Maden I, Kazak Z, Onan U et al. (2011) The effect of low-level Laser therapy as an adjunct to non-surgical periodontal treatment. , J Periodontol; 82, 481-488.
- 13.Irace C, Scavelli F, Carallo C, Serra R, Cortese C et al. (2009) Body mass index, metabolic syndrome and carotid atherosclerosis. , Coron Artery Dis; 20, 94-99.
- 14.Löe H. (1967) . The Gingival Index, the Plaque Index and the Retention Index Systems.J Periodontol;38:Suppl: 610-616.
- 15.Silness J, Löe H. (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. , Acta Odontol Scand; 22, 112-135.
- 17.Carallo C, Fortunato L, de Franceschi MS, Irace C, Tripolino C et al. (2010) Periodontal disease and carotid atherosclerosis: are hemodynamic forces a link? Atherosclerosis;. 213, 263-267.
- 18.Lopes B M, Marcantonio R A, Thompson G M, Neves L H, Theodoro L H. (2008) Short-term clinical and immunologic effects of scaling and root planing with Er:YAG Laser in chronic periodontitis. , J Periodontol; 79, 1158-1167.
- 19.Schwarz F, Sculean A, Georg T, Reich E. (2001) Periodontal treatment with an Er: YAG Laser compared to scaling and root planning. A controlled clinical study. , J Periodontol; 72, 361-367.
- 20.Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N et al. (2009) Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. , Lasers Med Sci; 24, 681-688.
- 21.Pejcic A, Kojovic D, Kesic L, Obradovic R. (2010) The effects of low level Laser irradiation on gingival inflammation. , Photomed Laser Surg; 28, 69-74.
- 22.Qadri T, Miranda L, Tunér J, Gustafsson A. (2005) The short-term effects of low-level Lasers as adjunct therapy in the treatment of periodontal inflammation. , J Clin Periodontol; 32, 714-719.
- 23.Calderín S, García-Núñez J A, Gómez C. (2013) Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated Laser phototherapy in chronic periodontitis. , Lasers Med Sci; 28, 157-166.
- 24.Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F et al. (2008) Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. , J Periodontol; 79, 1638-1644.
- 25.Lai S M, Zee K Y, Lai M K, Corbet E F. (2009) Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne Laser in the treatment of moderate to advanced periodontal disease: a pilot study. , Photomed Laser Surg; 27, 287-93.
- 26.Makhlouf M, Dahaba M M, Tunér J, Eissa S A, Harhash T A. (2012) Effect of adjunctive low level Laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. , Photomed Laser Surg; 30, 160-166.
- 27.Lui J, Corbet E F, Jin L. (2011) Combined photodynamic and low-level Laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. , J Periodontal Res; 46, 89-96.
- 28.Dukić W, Bago I, Aurer A, Roguljić M. (2013) Clinical effectiveness of diode Laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. , J Periodontol; 84, 1111-1117.
- 29.Obradović R, Kesić L, Mihailović D, Jovanović G, Antić S et al. (2012) Low-level Lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. , Diabetes Technol Ther; 14, 799-803.
- 30.Dinarello C A, Simon A, JW van der Meer. (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 11, 633-652.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Photochemistry and Photobiology (2021) Crossref
Photochemistry and Photobiology (2020) OpenAlex
Journal of Dentistry And Oral Implants (2016) OpenAlex
