Abstract
Thyroid carcinomas encompass a wide spectrum ranging from differentiated thyroid carcinoma (DTC) to poorly differentiated (PDC) and anaplastic thyroid carcinoma (ATC). DTC of both follicular (FTC) and papillary (PTC) types can progress to PDC and AC. The aim of our study was to evaluate if there is differential microRNA (miRNA) expression in various tumor subtypes during this progression. The miRNA profile of differentiated carcinomas (Follicular and Papillary) and ATC were compared with that of PDCs either by itself or in a background of differentiated carcinomas and anaplastic carcinomas. Unsupervised hierarchical clustering analysis revealed that FTC and PDC tend to cluster together in the absence of ATC. Interestingly, in cases with presence of all components i.e. FTC, PDC and ATC, the miRNA profile of poorly differentiated component clusters with that of the Anaplastic carcinoma component. miR-494 and miR-125a-5p were found to be differentially regulated in tumors with an anaplastic component and even the well-differentiated component (FTC) of these tumors were found to be aligned with the anaplastic profile. In addition, we also discovered some differentially regulated miRNAs in follicular variant of papillary thyroid carcinoma as compared to follicular thyroid carcinoma (miR-486-5p and miR-31).
Author Contributions
Academic Editor: Anna Brozyna, Department of Tumor Pathology and Pathomorphology Collegium Medicum Nicolaus Copernicus University, Oncology Center Franciszek Lukaszczyk Memorial Hospital
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Madhu P Menon, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Micro-RNAs (miRNAs) are small non-coding RNAs of approximately 21-25 nucleotides that negatively regulate gene expression 1, 2, 3. It is one of the largest gene families accounting for approximately 1% of the genome and regulate approximately 1/3rd of all human genes 4, 5, 6. Micro-RNAs have been demonstrated to have multiple roles in regulating embryonic development, proliferation, apoptosis, etc. It has also emerged as an important modulator of oncogenesis and have been demonstrated to be deregulated in various hematological neoplasms, prostate cancer, thyroid neoplasms, lung cancer, pancreatic and colo-rectal tumors etc 5.
Thyroid carcinoma, which constitutes more than 98% of thyroid malignancies accounts for 1% of all human cancers 7. Differentiated thyroid carcinoma (DTC) of both follicular (FTC) and papillary type (PTC) may progress to poorly differentiated carcinoma (PDC) which is a heterogeneous group that may either present as insular carcinoma or a group of less well-defined morphologic phenotype. It is not uncommon to see foci of insular growth associated with differentiated thyroid carcinoma of both papillary and follicular types. Yamashita et al. in a series of 82 follicular carcinoma reported presence of an insular component in 8 (10%) and furthermore presence of insular component was an independent risk factor for distant metastasis 8. PDC is associated with a prognosis worse than differentiated thyroid carcinoma but better than anaplastic thyroid carcinoma (ATC).
Micro-RNAs have been studied extensively in the context of thyroid carcinomas, especially well differentiated thyroid carcinomas 9, 10, 11, 12, 13, 14, 15. These studies have also addressed the various diagnostic and prognostic implications of micro-RNAs in thyroid carcinomas. Recently, Nikiforva et al showed that miR-187 was the most up-regulated miRNA in tumors harboring RET/PTC rearrangements and RAS mutations, however it’s expression is much lower in tumors with BRAF mutation 10. In the same study, it was also shown that hierarchial clustering revealed four distinct clusters of miRNAs; oncocytic follicular tumors, conventional follicular tumors, papillary carcinoma and medullary carcinoma 10. Interestingly, in the four cases of poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma that were studied, no distinct miRNA clustering was observed 10. However, larger studies need to be done with respect to poorly differentiated thyroid carcinoma to identify specifically modulated miRNAs which could have diagnostic or rognostic implications.
The goal of this study was to analyse specifically the global miRNA profile in poorly differentiated carcinomas of thyroid as well as study changes that might occur in the context of progression of well-differentiated thyroid carcinomas to poorly differentiated and anaplastic carcinomas.
Materials and Methods
Case Selection
A search of the pathology archives at our institution over an 18-year period (1990-2008) retrieved 28 cases of PDC. Tissue was available on 23 cases for the study, which included 15 cases of PDC with DTC, and 3 cases with ATC in addition to FTC and PDC. The DTC component on these 15 cases included FTC (7), follicular variant of papillary carcinoma FVPC (6) and PTC (2). In addition 6 cases were pure PDC and 2 pure ATC. Seventeen cases of DTC (9 FTC, and 8 PTC) only were also selected from the same period.
RNA Isolation
Formalin fixed paraffin embedded sections (FFPE) were deparaffinized and individual tumor areas were dissected by laser capture microdissection (Arcturus PixCell II LCM).. Total RNA was isolated using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion ®, Grand Island, NY) and reconcentrated using miRNeasy mini kit (Qiagen, Valencia, CA) or were directly enriched from FFPE sections using miRNeasy FFPE kit (Qiagen, Valencia, CA) per manufacturer’s protocol. Following this, quantitation was done using Nanodrop 2000 (Thermo scientific, Wilmington, DE). The samples with similar individual tumor components were pooled as described below.
miRNA Expression Profiling
RNA was reverse transcribed into cDNA using miRNA-specific stem-looped primers (Megaplex™ RT primers, Applied biosystems, Grand Island, NY) and preamplified as per manufacturer’s protocol. Subsequently, miRNA profiling was done by qRT-PCR with the TaqMan Human MicroRNA Array A v2.0 card on Applied biosystems 7900HT real-time PCR system as recommended by manufacturer.
qRT-PCR Replicate Studies
Individual microRNAs were assayed using replicate tumor type samples using TaqMan microRNA assay protocol (Applied biosystems, Grand Island, NY). miRNA expression levels were calculated by relative quantitation using Biorad CFX96™ Real-Time PCR detection system (Bio-Rad Labs, Hercules, CA).
Data Analysis and Statistical Analysis
Data analysis for miRNA expression profiling was done using Realtime StatMiner® software (Integromics, Philadelphila, PA). Briefly, Small nucleolar RNA RNU6B and RNU48 were used as endogenous controls for normalization. Relative quantity (RQ, fold-change compared to normal) was calculated using the ΔΔCT method. Log10RQ values were used for unsupervised hiearchial clustering with Cluster 3.0 and Java-tree view software 16, 17. This was also used to select top candidate miRNAs for further validation in replicate tumor tissue via qRT-PCR. For the individual miRNA qRT-PCR analysis, the Ct values were normalized to endogenous small nucleolar RNA RNU6B and fold change of expression was determined by 2−ΔΔCT method. Student’s t test was used to assay significance of fold-changes between samples.
Results and Discussion
Cases of thyroid carcinoma were selected which either had a well-differentiated component, a mix of two tumors (well-differentiated and poorly differentiated, set B) or a mix of three (follicular carcinoma, poorly differentiated and anaplastic carcinoma, set A) (Figure 1). The workflow is depicted in Figure 2. Subsequent to dissection of individual tumor components, individual RNA was isolated and pooled for the respective representative samples, reverse transcribed and cDNA amplification was carried out. The samples were eventually loaded onto Taqman Human miRNA Array A v2.0 microfluidics card and real time amplification was performed (Figure 2). Data analysis was subsequently performed with Realtime statminer.
Figure 1.Representative histologies of well-differentiated thyroid carcinomas (follicular & papillary thyroid carcinomas, 100x and 200x magnification respectively), poorly differentiated thyroid carcinoma (100x) and anaplastic thyroid carcinoma (400x) are shown. Cases were selected which either had a well-differentiated component, a mix of two tumors (well-differentiated and poorly differentiated, set B) or a mix of three (follicular carcinoma, poorly differentiated and anaplastic carcinoma, set A)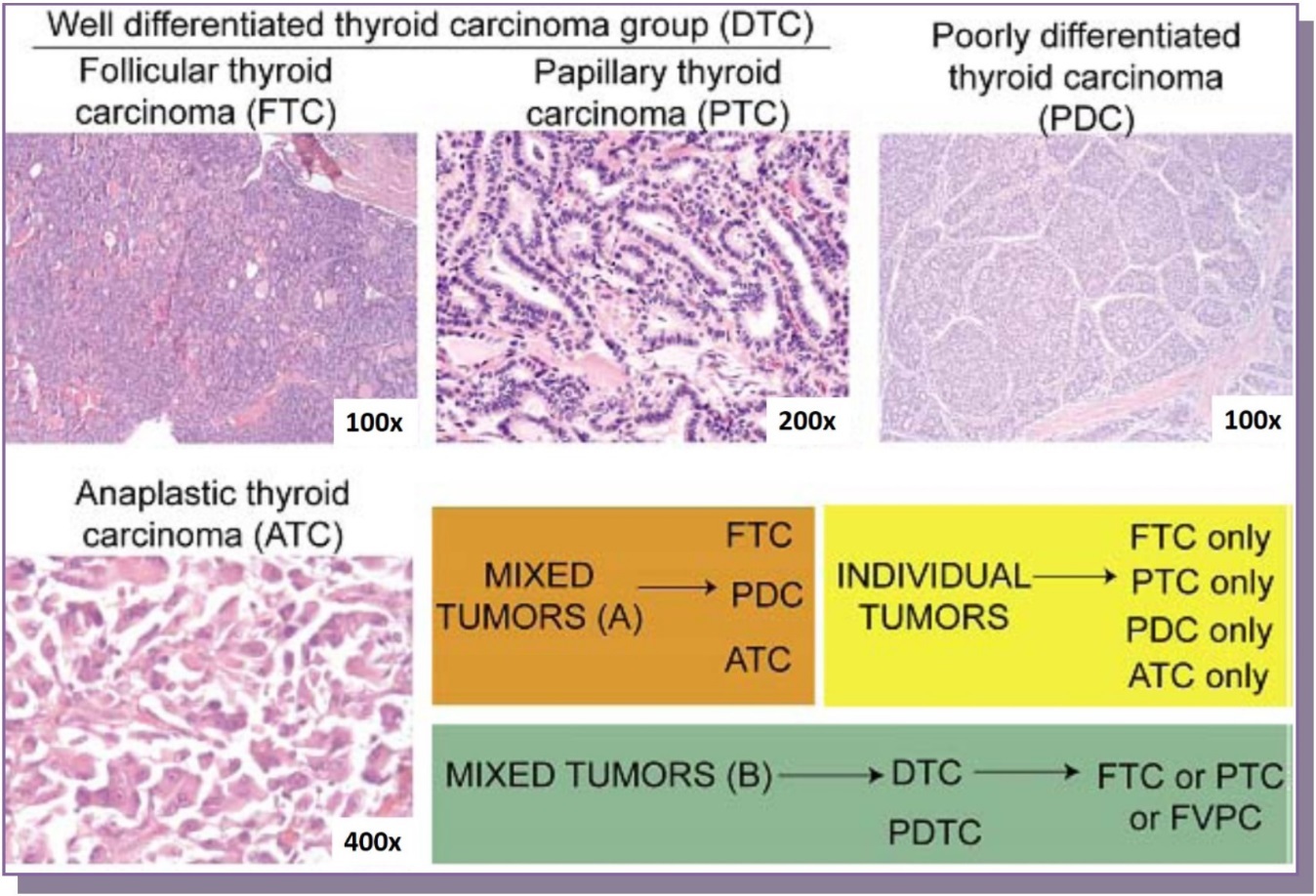
Figure 2.Subsequent to dissection of individual tumor components, RNA was isolated, reverse transcribed and cDNA amplification was carried out. Following loading of Taqman Human miRNA Array A v2.0 microfluidics card, real time amplification was performed on ABI 7900 HT platform. Data analysis was done with Realtime statminer, Cluster 3.0 and Java Tree view.
Unsupervised hierarchial clustering was performed with the help of Cluster 3.0 and Java-tree view software 16, 17. These revealed several interesting observations. When observing tumors with no mixed components (Figure 3B), the anaplastic carcinomas seemed to form a distinct cluster of their own as compared to the well-differentiated carcinomas which clustered within themselves on a global scale. Interestingly, Comparison of miRNA of set B cases with well differentiated carcinomas demonstrates that poorly differentiated carcinomas either in a background of follicular carcinoma or papillary carcinoma clusters together (separate from anaplastic carcinoma only or poorly differentiated carcinoma in a background of anaplastic carcinoma). So, it seems that poorly differentiated carcinomas are closer to their well-differentiated components in the absence of an anaplastic carcinoma component, while if poorly differentiated carcinoma component is present in the background of follicular carcinoma and anaplastic carcinoma, the miRNA profile of poorly differentiated carcinoma seems to align with that of anaplastic carcinoma. In addition, samples were also included of follicular component either dissected from a mixed tumor B (Follicular carcinoma + poorly differentiated carcinoma) or mixed tumor C (Follicular carcinoma + poorly differentiated carcinoma + Anaplastic carcinoma) and the miRNA profile seemed to align with the anaplastic carcinoma group (data not shown). In addition, poorly differentiated carcinoma miRNA profile even in the background of papillary thyroid carcinoma aligned with that of the “well-differentiated” group (Figure 3A). Unfortunately, we did not have cases of papillary thyroid carcinoma with both poorly differentiated and anaplastic component to compare.
Figure 3.A Comparison of miRNA of set B cases with WTC demonstrates that PDC in a background of either FC or PTC clusters together (separate from ATC only and PDC in a background of ATC). C, FTC, PTC and PDC cluster together separate from ATC.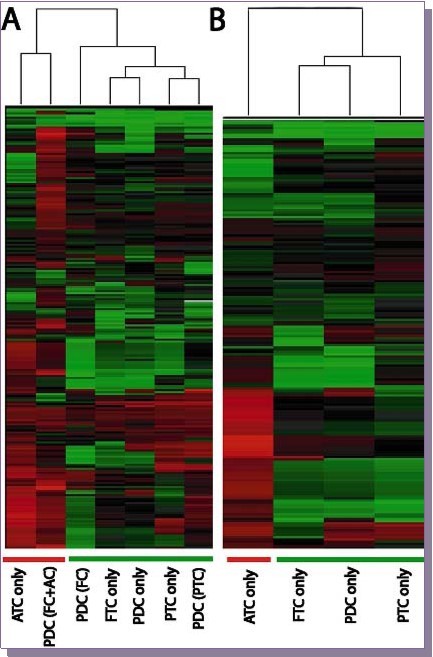
Specifically one of the goals was to select a group of miRNAs that were differentially modulated in the anaplastic carcinoma group and repeat qPCR studies with replicate samples. As opposed to the original profiling experiments, where samples were pooled, individual samples in this case were used for analysis. The goal was to locate specific miRNAs that were preferentially differentially regulated in follicular carcinomas in the background of anaplastic carcinoma as compared to follicular carcinomas which haven’t progressed to anaplastic carcinoma. miR-494 was found to be preferentially upregulated in FTC component of a mixed tumor which had progressed to anaplastic carcinoma (p<0.1) as compared to FTC in the background of PDC (FC/PDC) or poorly differentiated carcinoma (PDC) only samples and poorly differentiated carcinoma in the background of follicular carcinoma (PDC/FC) (Figure 4A). Similarly, a trend in the opposite direction was observed formiR-125a-5p (Figure 4B). However, this was statistically not significant. We were also interested in discovering miRNAs that would be differentially regulated in FVPC as compared to FTC. Specifically, miR-486-5p (Figure 4C) was preferentially upregulated in FTC sample as compared to FVPC and PTC (p<0.1) while miR-31 (Figure 4D) showed a trend in the opposite direction with upregulation in FVPC and PTC as compared to FTC (p<0.1).
Figure 4.qRT-PCR studies in replicates (Fold change values normalized to normal thyroid tissue) are shown. Differential regulation of miR-494 (A) and miR-125a-5p (B) in follicular carcinoma in the background of either anaplastic carcinoma (FC/AC) or poorly differentiated carcinoma (FC/PDC) along with follicular carcinoma (FC) and poorly differentiated carcinoma (PDC) only samples and poorly differentiated carcinoma in the background of follicular carcinoma (PDC/FC). Differential regulation of miR-486-5p (C) and miR-31 (D) in Follicular carcinoma only sample as compared to follicular variant of papillary thyroid carcinoma and poorly differentiated carcinoma.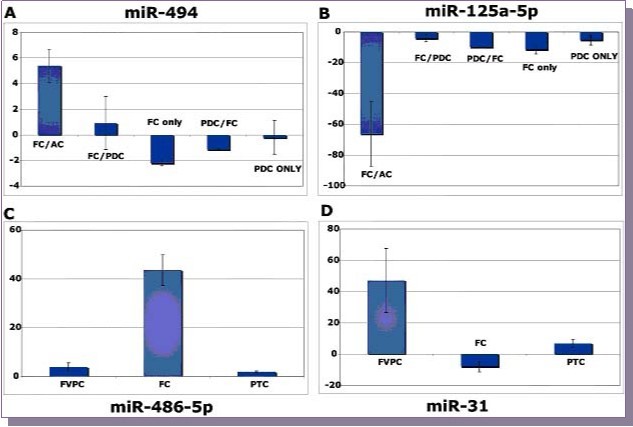
There are few studies describing miRNA studies in PDCs. Schwertheim et al. described the expression of two different sets of microRNAs (‘set 1’: miRNA-146b, -181b, -21, -221 and -222, upregulated in PTCs; ‘set 2’: miRNA-30d, -125b, -26a, -30a-5p and let7d, downregulated in ATC) 18. These were compared in 15 PDCs, nine PTCs and nine ATCs and Set 1’ microRNA expression was found to be slightly increased in PDCs but did not significantly differ from normal thyroid tissue. miRNAs of ‘set 2’ were expressed at low levels in PDCs and ATCs but significantly upregulated in PTCs 18. Also, Nikiforova et al. investigated the microRNA expression of four PDCs and found miR-181b, -221, -222 and -146b to be upregulated when compared to normal thyroid 10. Through our study, we describe the global miRNA profile of PDCs in various contexts (especially in the context of mixed tumors) and we observe several interesting trends . While qRT-PCR confirmation of only some of the targets has been achieved, discovery of more differentially regulated miRNAs and their verification in replicate samples is in progress.
References
- 1.Bartel D P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. , Cell 116(2), 281-97.
- 3.Ambros V. (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. , Cell 113(6), 673-6.
- 4.Kim V N. (2005) MicroRNA biogenesis: coordinated cropping and dicing. , Nat Rev Mol Cell Biol 6(5), 376-85.
- 5.Esquela-Kerscher A, Slack F J. (2006) Oncomirs - microRNAs with a role in cancer. , Nat Rev Cancer 6(4), 259-69.
- 6.Stefani G, Slack F J. (2008) Small non-coding RNAs in animal development. , Nat Rev Mol Cell Biol 9(3), 219-30.
- 7.Hundahl S A. (2000) Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during. , U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 89(1), 202-17.
- 8.Yamashita H.et al.(2005). Significance of an insular component in follicular thyroid carcinoma with distant metastasis at initial presentation. , Endocr Pathol 16(1), 41-8.
- 9.Weber F. (2006) A limited set of human MicroRNA is deregulated in follicular thyroid carcinoma. , J Clin Endocrinol Metab 91(9), 3584-91.
- 10.Nikiforova M N. (2008) MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. , J Clin Endocrinol Metab 93(5), 1600-8.
- 11.He H. (2005) The role of microRNA genes in papillary thyroid carcinoma. , Proc Natl Acad Sci U S A 102(52), 19075-80.
- 12.Pallante P. (2006) MicroRNA deregulation in human thyroid papillary carcinomas. , Endocr Relat Cancer 13(2), 497-508.
- 13.Takakura S. (2008) Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. , Cancer Sci 99(6), 1147-54.
- 14.Visone R. (2007) Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. , Oncogene 26(54), 7590-5.
- 15.Ledent C. (1991) Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. , Endocrinology 129(3), 1391-401.
