In Vitro Cytoprotection of Resveratrol against H2O2-Induced Oxidative Stress and Injury in Astrocytes
Abstract
Oxidative stress mediated neural cell death is thought to be involved in the progression of secondary cell injury following brain trauma. Agents that can block oxidative stress-related injury could be potential therapies for TBI. Resveratrol, a polyphenol found in plants and red wine, is cytoprotective due to its potent antioxidant activities. To further understand how resveratrol could affect oxidative stress-induced injury, we hypothesized that the cytoprotective activities of resveratrol could be dose-dependent. In this study, resveratrol-induced cytoprotection was evaluated in cultured astrocytes. Primary rat astrocytes were cultured in T-75 flasks to a confluence of 80% before being plated onto 96-well plates. After 24 hours of acclimation, astrocytes were treated with various doses of hydrogen peroxide (H2O2) (0.1, 0.25, 0.5 and 1 µM) and resveratrol (25, 50, 75, 100 µM), respectively. Cell viability was determined 24 hours later using Alamar Blue Assay. Treatment of astrocytes with 0.5 mM H2O2, left 65% of astrocytes non-viable whereas treatment of astrocytes with 0.1 mM H2O2 had no effect on astrocytes viability; whereas 1 mM, H2O2 caused total loss of astrocyte viability. Resveratrol treatment at 75 µM and 100 µM has reduced 0.5 mM H2O2-induced cytotoxicity in astrocytes by 50%. Immunostaining with GFAP also confirmed these findings about the cytoprotective effects of resveratrol in astrocytes exposed to H2O2. These results suggest that resveratrol could be a potential neuroprotective agent in TBI due to its antioxidant properties. Further studies are needed to evaluate the long- term effects of resveratrol in animal models of TBI.
Author Contributions
Academic Editor: Hyemyung Hseo, Department of Molecular & Life Sciences, Hanyang University, Republic of Korea.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Guoqiang Xing, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Traumatic brain injury (TBI) is the leading cause of death in young people. So far there is no FDA approved drug for TBI. Because oxidative stress-induced neural cell death is thought to be involved in the secondary injury and poor outcome of TBI, plant and fruit-derived antioxidants could be potentially used for treating TBI. Increasing evidence suggests that resveratrol is such a promising neuroprotective agent. Yet their safety and toxicity should be evaluated before clinical application. Resveratrol (3, 5, 4’-Trihydroxy-trans-stilbene) is a polyphenol found in grapes, berries, peanuts and most abundantly in red wines. Resveratrol is a strong antioxidant that can shield cells from free radical-induced oxidative stress and damage, with strong cardioprotective and anti-inflammatory properties1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Recent studies have shown that resveratrol is also neuroprotective14, 15 and can stimulate lipid and glucose metabolism which are the metabolic source of reactive oxygen species and oxidative damage16, 17, 18, 19, 20, 21, 22, 23. Because different concentrations of resveratrol may be present in red wine, it is important to know the minimal dose range of resveratrol that can be cytoprotective without toxicity and other side effects.
Astrocytes are the most abundant cell types present in the brain. The main function of astrocytes, among others, is to protect neurons from injury by providing antioxidants and energy precursors such as glutathione and lactate 24, 25, 26, 27. Based on the literature and the observation that consumption of low to moderate amounts of red wine could promote health in humans, we hypothesized that resveratrol administered at relatively low range of concentrations could be cytoprotective. By studying the cytoprotective and cytotoxic effects of resveratrol in astrocytes, we hope to identify the safe dose of resveratrol that could be a guidance for future studies of resveratrol neuroprotection in neurons and in animal model of TBI.
Materials and Methods
Materials
Resveratrol, RPMI 1640 medium with phenol red, 200 proof 100% ethanol (EtOH), and 30% hydrogen peroxide (H2O2) were purchased from Sigma-Aldrich (MO, USA). Dulbecco’s Modified Eagle Serum (DMEM), fetal bovine serum, Gentamicin, phosphate buffered saline, trypsin, HyPur Sterile cell culture grade water and alamarBlueÒ were purchased from Life Technologies (San Diego, CA).
Cell Culture
Primary rat astrocytes were derived from postnatal rat pups as reported 28 and sub-cultured in T-75 flasks in 15mL of DMEM for two to three days until they grew to a confluence of at least 80%. 10% FBS and 10ug/mL of Gentamicin were added to the media before plating and feeding the cells used in the study. After the old media was aspirated out, each flask was washed with PBS before the cells were treated with 3.5mL of trypsin to gently detach the cells from the flasks. After one to five minutes, the trypsin was deactivated by adding 3.5 mL of fresh growth media to the flasks. The suspension of deactivated trypsin and cells was transferred to a 15 mL conical tube and centrifuged at 1000 rpm for 5 minutes. After removing the supernatant, 5 mL of fresh media was added to the tube to suspend the pellet. The cell viability was determined using a Vi-Cell XR cell viability analyzer (Beckman Coulter). At any given point in the ten-week study, cells were no less than 92% viable when transferred from the flasks to the 96-well plates. In order to have the 96-well plates ready for treatment the following day, at least 3.5 x 105 cells were plated per well. The remaining cell suspension was plated into new T-75 flasks.
All the hydrogen peroxide and ethanol solutions used in this experiment were made fresh for each experiment. A stock working concentration of 0.25% EtOH was prepared from the 100% EtOH. A 100 mM stock concentration of resveratrol was made by dissolving it in 0.25% ethanol. A new stock of resveratrol was made every two weeks. According to our observation, the 0.25% ethanol did not alter the condition of the astrocytes when compared to the control media used in each study.
Treatment
After all the solutions were prepared, the cells growing in 100 μl of the culture medium were treated with desired amount of stock solutions. For the first three experiments, cells were given 24 hours to respond to the injury-inducing reagent H2O2, and the protective agent resveratrol. Some of the trials were designed as time-course studies where cell injury was induced first with H2O2 for 90 minutes and then followed by resveratrol treatment for three days. Other trials were designed as dose-response studies with H2O2 and resveratrol independent of each other, to understand the specific effects of each on the cells. Each experiment was repeated 6 to 8 times with triple repeats per sample per experiment.
Cell Viability Analysis
The cells were viewed under a light microscope at the beginning of each trial and every 24 hours thereafter until the reaction became evident at which point the plate would be treated with 20 mL of Alamar Blue. Twenty-four hours after Alamar Blue treatment, a reading was taken using a Multi-Skan plate reader [Thermolyne Laboratories). Healthy cells metabolized the blue-colored reagent and turned it pink. Nonviable or damaged cells were unable to metabolize the substance and therefore remained blue [Life Technologies, CA).
Immunocytochemistry Analysis
Cultured astrocytes were fixed with 4% paraformaldehyde (pH 7.4) for 15 minutes, then washed with PBS, and incubated with 0.1% triton X-100 in PBS for 30 minutes in blocking solution [PBS containing 4% normal goat serum 0.05% triton X-100) for 30 min. Cells were incubated with primary antibody - rabbit GFAP [glial fibrillary acidic protein; DAKO Cytomation, Glostrup, Denmark, 1:1000) overnight. After PBS wash, cells were incubated with FITC conjugated donkey anti-rabbit secondary antibody(1:500) [Jackson ImmunoResearch Lab, Inc.) for one hour. Cell slides were counterstained with DAPI (Vectashield DAPI, Vector Labs). Fluorescence was visualized with a Zeiss Axiom observer Z1 inverted microscope (NPC) and Zeiss LSM 510 laser scanning co focal microscope.
Data analysis: Group means (mean +S.D) in cell viability were compared between different treatment methods and between different doses using one-way-ANOVA followed by post hoc Bonferroni test. A p value < 0.05 was considered statistically different.
Results
Dose response studies with H2O2 suggest that increasing doses of H2O2 significantly reduced the viability of astrocytes. The cell-viability data showed that 0.1mM H2O2 was ineffective in damaging the astrocytes whereas 1mM H2O2 treatment caused almost total loss of astrocyte viability that no viable cells were left to metabolize alamarBlueÒ added to the system after the H2O2 injury (Figure 1). Treatment of the cells with 0.5mM H2O2, resulted in 35% viable astrocytes compared with controls (Figure 1). Treatment with H2O2 at other three concentrations (0.1, 0.25 and 1 mM) induced different levels of cell injury leaving approximately 50% of the viable astrocytes.
Figure 1.Treatment of hydrogen peroxide (H2O2) for 24 hours dose-dependently suppressed the viability of cultured rat astrocytes [as indicated by the presence of alamarBlueÒ). H2O2 at 0.1 mM was not effective whereas H2O2 at 1 mM was very effective in inhibiting cell viability. Treatment of astrocytes with 0.5mM H2O2 left 35% of the cells alive.
Figure 2.Resveratrol at the concentration of 25, 50, 75 and 100 µM produced no cytotoxicity in cultured rat astrocytes as shown in the alamarBlue viability assay. Resveratrol at the concentration of 125, 150, 175 and 200 µM enhanced the viability of astrocytes ( p<0.05 each). *, p<0.05, viability of resveratrol-treated astrocytes vs. viability of naïve astrocytes (100%).
To evaluate the potential cytoprotective and harmful effects of resveratrol, the astrocytes were treated with different concentrations of resveratrol (Figure 2). Resveratrol in the micromolar concentration range [25-100 µM) did not affect significantly on astrocytes viability compared to the non-treatment group [100%) (Figure 2). Resveratrol at the 125, 150, 175 µM concentration significantly promoted the viability of astrocytes when compared to the baseline values (P<0.05)(Figure 2).
Figure 3.Simultaneous treatment of cultured astrocytes with 0.5 mM H2O2 and different dose of resveratrol resulted in a resveratrol dose-dependent increase in cell survival. At the concentration of 75 µM, resveratrol increased the cell viability to about 50% of the control value, thereafter, the neuroprotective effect of resveratrol leveled off.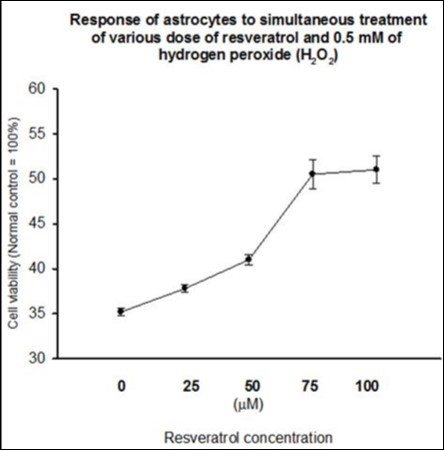
Twenty-four hours of simultaneous treatment of cultured astrocytes with 0.5 mM H2O2 and with increasing dose of resveratrol resulted in a resveratrol dose-dependent increase in cell survival (Figure 3). At the concentration of 75 µM, resveratrol increased the cell viability to about 50% of the control value, thereafter, the neuroprotective effect of resveratrol leveled off.
Figure 4.Resveratrol treatment protected astrocytes from H2O2 induced-injury. The left image shows the normal control astrocytes grown in DMEM media. The center image shows the astrocyte injury after 24 hours of 0.5mM H2O2 treatment. The cell became bluefish because the majority of the cells were dead as the result of H2O2 injury. The right image shows the astrocytes simultaneously protected with 75μM resveratrol treatment given at the onset of H2O2-induced injury. Resveratrol-protected cells were viable and able to metabolize alamar blue and turn it into pink color. All microscope photographs are in 10X magnification.
The astrocytes treated with 0.5 mM H2O2 treatment and protected with 75μM resveratrol were examined visually (Figure 4). Normal control astrocytes as seen on the left are raindrop-shaped and have very plump filaments. The base of the culture well is full of confluent and healthy cells. Astrocytes in the center picture represent the cells after 0.5mM H2O2-injury.
Hydrogen peroxide (H2O2) caused the cells to cluster around each other. The cells became hard balls with shriveled out filaments. They were dying and bluish as they were not viable to metabolize the alamar blue reagent. The image on the right shows the evidence of the cytoprotective effects of adding 75μM resveratrol to the 0.5 mM H2O2-injured cellsthat were more viable than and not as shriveled as the unprotected cells. However, these cells were somewhat unhealthy due to the H2O2 injury even though they were viable and metabolizing the alamar blue.
Figure 5.The effects of different order of resveratrol treatment (given 90 min before or 90 min after H2O2-induced injury) on cell viability were evaluated in astrocytes. Resveratrol administered 90 min after the initiation of 0.5mM H2O2–induced injury (black line) provided significantly better cytoprotection than resveratrol administered before the initiation of H2O2–induced injury (red line). Although all five concentrations of post-injury resveratrol (25, 50, 100, 150 and 200 µM) provided significant protection than the non-protected controls, resveratrol at 25 and 50 μM produced better cytoprotection than resveratrol at 100, 150 and 200 μM. **, P<0.01, differences in cell viability between resveratrol treatment given 90 min before, and 90 min after 0.5 mM H2O2–induced injury.aa, P<0.01, astrocytes with 25 and 50 μM resveratrol treatment showed better viability than astrocytes with 100, 150 and 200 μM resveratrol treatment given 90 min after 0.5mM H2O2–induced injury.
The effects of treatment order of resveratrol on astrocyte viability were examined in two studies, i.e. if resveratrol administered ahead of the H2O2-induced injury would provide better protection against the injury than resveratrol administered after the initiation of H2O2-induced injury. In the first study, five concentrations (25, 50, 100, 150 and 200 µM) of resveratrol were given to the astrocytes for 90 minutes before the treatment of 0.5mM H2O2 (black line in Figure 5). In the second study, 0.5mM H2O2 was given to the astrocytes for 90 min then followed by the resveratrol treatment (red line in Figure 5). The results show that H2O2–injury followed by resveratrol treatment better protected the cells than resveratrol treatment followed by H2O2 –injury. It also appears that the lower doses of resveratrol (25 and 50 µM) were more effective than high doses (100, 150 and 200 µM) of resveratrol for pre-existing H2O2–injury (P<0.01, respectively).
Figure 6.Immunocytochemistry analysis of GFAP expression (red color) in resveratrol-protected astrocytes after 24 h of 0.5 mM H2O2 injury (bottom panels). DAPI nuclear stain was used to visualize cell nuclei (blue color). Left images, astrocytes without resveratrol protection; middle images, astrocytes protected with 50 μM resveratrol, right images, astrocytes protected with 75 μM resveratrol.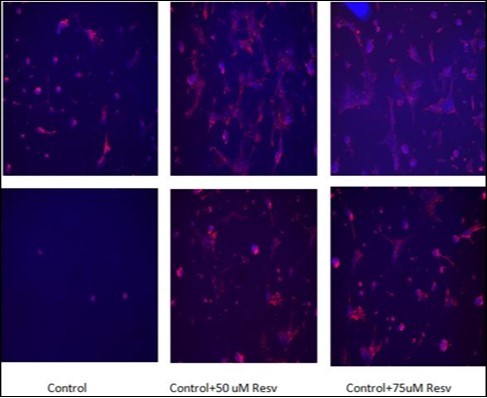
The cytoprotective effects of resveratrol in H2O2-treated astrocytes were further validated using immunocytochemistry methods. GFAP expression, a marker for mature astrocytes, was clearly shown in normal astrocytes but not in astrocytes after 24 h treatment with 0.5 mM H2O2 (Figure 6). Treatment of astrocytes with either 50 mM or 75 µM of resveratrol, however, resulted in significant cytoprotection of H2O2-treated astrocytes as reflected in the increased GFAP expression in the H2O2-treated astrocytes (Figure 6).
Discussion
We examined the effects of resveratrol treatment on cell viability in cultured rat astrocytes with and without H2O2 pre-treatment. Administration of resveratrol at the micro molar range (25-100 µM) did not affect the viability of naïve astrocytes whereas at the concentration of 125, 150, 175 and 200 µM, resveratrol enhanced the viability of astrocytes (6-8%, P<0.01). Treatment with H2O2 at the concentration of 0.5 mM and above resulted in a significant (>50%, P<0.01) reduction in cell viability in astrocytes. Administration of a low dose of resveratrol (between 25 µM and 200 µM) started 90 min after 0.5 mM H2O2-initiated cytotoxicity but not before H2O2 administration significantly protected astrocytes from H2O2-induced oxidative stress and injury and reduction in cell viability in astrocytes. Thus, post-injury treatment of astrocytes with a relatively low dose of resveratrol is cytoprotective in astrocytes.
Several recent studies have shown that: 1) pre-treatment with low concentration of resveratrol (0.1-10 µM) for 1 hour was cytoprotective against ethanol-induced cytotoxicity in astrocytes whereas treatment with higher concentrations of resveratrol (50-100 µM) enhanced ethanol-induced cytotoxicity 29 2) treatment with resveratrol (10, 30, 50 µM) dose-dependently reversed H2O2 (100 mM) -induced reduction in the viability of endothelia cells and reduction in micro RNA (miR-126) expression30 Furthermore, the protection of endothelial cells against oxidative injury by resveratrol treatment has been linked to the activation of PI3K/Akt by miR-126 30.
It has been reported that resveratrol treatment (200 mg/kg, i.p.) three time per day for three days significantly reduced neuronal cell death and improved functional recovery in the rat model of spinal cord injury 31. Resveratrol treatment reversed the reduction of superoxide dismutases (SOD) activity and increase of malondialdehyde [MDA) level as well as the expression of inflammatory cytokines including IL-1b, IL-10, TNF-a, and myeloperoxidase (MPO) after spinal cord injury, suggesting anti-oxidation, anti-inflammation and anti-apoptosis effects of resveratrol 31. Other study showed that daily resveratrol treatment (25 mg/kg, i.p.) for three weeks protected Lipid peroxides-induced cerebral oxidative stress in obesity rat brain through upregulating the suppressed expression of SOD, catalase, glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase 32, suggesting a protective effect of resveratrol by preventing oxidative damage in brain tissues with dysregulated lipid metabolism.
Studies have also shown that that chronic administration of resveratrol to young-adult rats significantly protected the olfactory cortex and hippocampus from the damage caused by systemic injection of excitotoxin kainic acid 33 and protected rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway 14. Trans-resveratrol has been reported to inhibit excitatory synaptic transmission and voltage-activated potassium currents in rat hippocampal neurons 15, 34, inhibit inflammatory responses in cultured LPS-stimulated microglial cells 11, and reduce early inflammatory responses induced by status epilepticus via the mammalian target of rapamycin (mTOR) signaling pathway 35. Thus, multiple mechanisms may be involved in resveratrol-related neuroprotection.
One recent prospective cohort study of 783 community-dwelling 65 years or older adults suggests a relationship between urinary resveratrol excretion and mortality 36. During a 9-year follow-up, 268 (34.3%) of the participants died. From the lowest to the highest quartile of baseline total urinary resveratrol metabolites, the proportion of participants who died from all causes was 34.4%, 31.6%, 33.5%, and 37.4%, respectively (P = .67). Participants in the lowest quartile had a hazards ratio for mortality of 0.80 (95%CI, 0.54-1.17) compared with those in the highest quartile of total urinary resveratrol excretion. While the results are interesting and appear to contradict the cytoprotective effect of resveratrol, it is noted that alcohol consumption, smoking, cognitive and physical activity were highest among participants in the highest quartile of total urinary resveratrol metabolites compared with the lower quartiles. In addition, participants in the highest quartile of total urinary resveratrol excretion had the highest incidents of fasting diabetic hyperglycemia (14.4% vs. an averaged 8.1% of other combined quartiles). Because diabetic nephropathy (as reflected by increased urinary protein and macro nutrients loss) is a common cause of increased mortality 37, the higher urinary resveratrol excretion could have resulted from a compromised kidney function rather than a reduced dietary intake of resveratrol suggested by the authors 36. Moreover, the significant correlation (r=0.67, P<0.001) between alcohol consumption and the total urinary resveratrol concentrations suggests that alcohol or smoking may be involved in urinary resveratrol excretion and increased mortality. Further analysis of the data without including the people with fasting diabetic hyperglycemia or study with known serum and urinary creatinine and resveratrol levels (as the indicator of kidney function and dietary intake) may better evaluate the role of resveratrol in age-related mortality.
There are limitations of this pilot study. We only examined the effects of resveratrol in cultured astrocytes but not neurons or oligodendrocytes which may have different sensitivity to H2O2 and resveratrol. Although the focus of this study is to evaluate the minimal safe dose of resveratrol in oxidatively stressed astrocytes, the high dose range of resveratrol, should also be evaluated in future in vitro and in vivo studies for potential cytotoxic effect on neurons and astrocytes before any clinical trials. It is still not clear why resveratrol administered after the onset of H2O2-induced injury was more effective than resveratrol administered before the initiation of H2O2- injury in reducing the astrocyte cytotoxicity. It is possible that increased resveratrol metabolism in normal uninjured cells is responsible for a rapid turnover and thus reduced effects of resveratrol.
In conclusion, low doses of resveratrol administered after the initiation of H2O2-induced injury protected astrocytes against cytotoxicity, without showing detrimental effects in normal astrocytes. Since resveratrol is a relatively safe and plentiful plant-derived dietary supplement, and because so far there is no proven effective agents that can block the secondary injury in patients with severe TBI, further studies are warranted to verify the long-term therapeutic effects of resveratrol in animal model of TBI.
Acknowledgment
This research was supported by an Exploratory grant to PS.
Daniel Xing proofread this manuscript.
References
- 1.CS Peritore, Ho A, BK Yamamoto, SE Schaus. (2012) Resveratrol attenuates L-DOPA-induced hydrogen peroxide toxicity in neuronal cells. , Neuroreport 23, 989-994.
- 2.Tiwari V, Chopra K. (2013) Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative-nitrosative stress and inflammatory cascade in the adult rat brain. , Neurochemistry international 62, 861-869.
- 3.Selvaraj S, Mohan A, Narayanan S, Sethuraman S, UM Krishnan. (2013) Dose-dependent interaction of trans-resveratrol with biomembranes: effects on antioxidant property. , Journal of medicinal chemistry 56, 970-981.
- 4.Quincozes-Santos A, LD Bobermin, Latini A, Wajner M, DO Souza et al. (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PloS one. 8-64372.
- 5.LM Vieira de Almeida, CC Pineiro, MC Leite, Brolese G, RB Leal et al. (2008) Protective effects of resveratrol on hydrogen peroxide induced toxicity in primary cortical astrocyte cultures. , Neurochemical research 33, 8-15.
- 6.Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F et al. (2013) Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association.
- 7.AA Aadam, JA Martin. (2013) Enteral stents in malignant bowel obstruction. , Gastrointestinal endoscopy clinics of North America 23, 153-164.
- 8.Paepe B De, Vandemeulebroecke K, Smet J, Vanlander A, Seneca S et al. (2014) Effect of resveratrol on cultured skin fibroblasts from patients with oxidative phosphorylation defects. , Phytotherapy research : PTR 28, 312-316.
- 9.Che R, Yuan Y, Huang S.Zhang A (2014): Mitochondrial dysfunction in the pathophysiology of renal diseases. , Am J Physiol Renal Physiol 306, 367-378.
- 10.Mohammadshahi M, Haidari F.Ghadiri Soufi F (2013): Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiology journal.
- 11.LM Zhong, Zong Y, Sun L, JZ Guo, Zhang W et al. (2012) Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PloS one. 7-32195.
- 12.DW Park, JS Kim, BR Chin, SH Baek. (2012) Resveratrol inhibits inflammation induced by heat-killed Listeria monocytogenes. , Journal of medicinal food 15, 788-794.
- 13.HR Vasanthi, RP Parameswari, DeLeiris J, DK Das. (2012) Health benefits of wine and alcohol from neuroprotection to heart health. , Front Biosci (Elite Ed) 4, 1505-1512.
- 14.Della-Morte D, KR Dave, RA DeFazio, YC Bao, AP Raval et al. (2009) Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. , Neuroscience 159, 993-1002.
- 15.ZB Gao, XQ Chen, GY Hu. (2006) Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. , Brain 1111, 41-47.
- 16.Momchilova A, Petkova D, Staneva G, Markovska T, Pankov R et al. (2014) Resveratrol alters the lipid composition, metabolism and peroxide level in senescent rat hepatocytes. Chemico-biological interactions. 207, 74-80.
- 17.Renaud J, Bournival J, Zottig X, MG Martinoli. (2014) Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. , Neurotoxicity research 25, 110-123.
- 18.NA Baker, English V, Sunkara M, AJ Morris, KJ Pearson et al. (2013) Resveratrol protects against polychlorinated biphenyl-mediated impairment of glucose homeostasis in adipocytes. , The Journal of nutritional biochemistry 24, 2168-2174.
- 19.Baczko I, Liknes D, Yang W, KC Hamming, Searle G et al. (2014) Characterization of a novel multifunctional resveratrol derivative for the treatment of atrial fibrillation. British journal of pharmacology. 171, 92-106.
- 20.Gomez-Zorita S, Treguer K, Mercader J, Carpene C. (2013) Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. Journal of physiology and biochemistry.
- 21.Zhang L, Zhou G, Song W, Tan X, Guo Y et al. (2012) Pterostilbene protects vascular endothelial cells against oxidized low-density lipoprotein-induced apoptosis in vitro and in vivo. Apoptosis : an international journal on programmed cell death. 17, 25-36.
- 22.Voloshyna I, SM Hussaini, AB Reiss. (2012) Resveratrol in cholesterol metabolism and atherosclerosis. , Journal of medicinal food 15, 763-773.
- 23.Zhang H, Morgan B, BJ Potter, Ma L, KC Dellsperger et al. (2010) Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: in vivo demonstration with magnetic resonance imaging. American journal of physiology Heart and circulatory physiology. 299 :. 985-994.
- 24.Armato U, Chakravarthy B, Pacchiana R, JF Whitfield. (2013) Alzheimer’s disease: an update of the roles of receptors, astrocytes and primary cilia (review). International journal of molecular medicine. 31, 3-10.
- 25.DM Garcia, JR Koke. (2009) Astrocytes as gate-keepers in optic nerve regeneration--a mini-review. , Comp Biochem Physiol A Mol Integr Physiol 152, 135-138.
- 26.DM Landis, TS Reese. (1981) Membrane structure in mammalian astrocytes: a review of freeze-fracture studies on adult, developing, reactive and cultured astrocytes. , J Exp Biol 95, 35-48.
- 27.SK Malhotra, TK Shnitka, Elbrink J. (1990) Reactive astrocytes--a review. , Cytobios 61, 133-160.
- 28.Sharma P, Karian J, Sharma S, Liu S, PD Mongan. (2003) Pyruvate ameliorates post ischemic injury of rat astrocytes and protects them against PARP mediated cell death. , Brain research 992, 104-113.
- 29.Gonthier B, Allibe N, Cottet-Rousselle C, Lamarche F, Nuiry L et al. (2012) Specific Conditions for Resveratrol Neuroprotection against Ethanol-Induced Toxicity. , Journal of toxicology.2012 : 973134.
- 30.XQ Sui, ZM Xu, MB Xie, DA Pei. (2014) Resveratrol Inhibits Hydrogen Peroxide-Induced Apoptosis in Endothelial Cells via the Activation of PI3K/Akt by miR-126. Journal of atherosclerosis and thrombosis. 21, 108-118.
- 31.Liu C, Shi Z, Fan L, Zhang C, Wang K et al. (2011) Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. , Brain research 1374, 100-109.
- 32.SD Rege, Kumar S, DN Wilson, Tamura L, Geetha T et al. (2013) Resveratrol protects the brain of obese mice from oxidative damage. Oxidative medicine and cellular longevity.2013:. 419092.
- 33.Virgili M.Contestabile A (2000): Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. , Neuroscience 281, 123-126.
- 34.ZB Gao, GY Hu. (2005) Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. , Brain research 1056, 68-75.
- 35.SJ Wang, QY Bo, XH Zhao, Yang X, ZF Chi et al. (2013) Resveratrol pre-treatment reduces early inflammatory responses induced by status epilepticus via mTOR signaling. , Brain research 1492, 122-129.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
International Journal of Molecular Sciences (2024) Crossref
International Journal of Molecular Sciences (2024) OpenAlex
Journal of Behavior Therapy And Mental Health (2016) OpenAlex
