Enhanced Alkaloid Production from Cell Culture System of Catharanthus roseus in Combined Effect of Nutrient Salts, Sucrose and Plant Growth Regulators
Abstract
Callus and biomass culture of Catharanthus roseus L. were established to check for the presence of total alkaloid and its subsequent yield. Various treatments like strength of nutrient salts, sucrose concentrations and combinations of plant growth regulators (PGR’s) were applied to both MS and B5 in agar as well as suspension medium to test their effects on enhanced alkaloid content and its yield. There was no significant difference in any of the observable parameters of fresh wt, dry wt, alkaloid content, production, productivity and yield if the culture were treated similarly in both types of media formulations (MS or B5 salts). Physical state (agar solidified or the liquid suspension) of the medium had significant effect on all the parameters particular on fresh wt, alkaloid content and yields. Although, the fresh wt. and dry wt. of biomass in suspension culture was 2-3 times less than that of callus obtained from agar medium. However, the alkaloid content and yield was 2-3 times higher in suspension culture compared to agar medium in similar treatments. The highest alkaloid content observed was 5.67mg/g dwt in B5 suspension medium containing 3% sucrose and modified with 0.5mg/l 2,4-Dichlorophenoxy acetic acid (2,4-D) + 1 mg/l Kinetin (KIN) + 2mg/l α- naphthalene acetic acid (NAA). The combined effects of these factors on the enhanced production of total alkaloids were expected to contain higher yield of anticancer vinblastine and vincristine in the cell suspension culture system.
Author Contributions
Academic Editor: Hammad Afzal, SZABIST, Karachi, Pakistan.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Malay Ranjan Mishra, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Plants are rich source of a number of secondary metabolites including the alkaloids with an estimated count of around 3000 different types till date 1. The indole alkaloid, also known as monoterpenoid indole alkaloids (MIA) or terpenoid indole alkaloids (TIA) are derived from strictosidine which interns obtained from tryptamine and a portion from iridoid secologanin 2, 3. TIA pathway depends on indole and terpenoid precursors supplied by two convergent branches of the primary metabolites the shikimate and the isopentenyl diphosphate pathways. It involves more than twenty enzymes located in cytosol, (pro)vacuoles and chloroplasts/plastids 4. These indole alkaloids such as antimalarial quinine from Chincona officinalis, the antineoplastic camptothecine from Camptothecaacuminta, the rat poison and tonic like strychnine from Strychnosnux-vomica, antihypertensive and tranquilizer resperine from Rauvolfia sp., and the widely investigated antitumor agents vinblastine and vincristine from Catharanthus roseus are now being prescribed in modern therapeutics to treat the diseases.
Catharanthus roseus L. (Apocynacea) also known as Madagascar periwinkle is one of the most extensively used and investigated medicinal plant 5. This is a major source of the highly important anti-cancer bisindole alklaoids i.e. vinblastine and vincristine 6, 7, 8, 9. These alkaloids are responsible for control of white cells in blood (an indicator of leukemia) thus acting as an anticancer drug by preventing mitosis in the metaphase stage when they bind to the tubulin thus inhibiting the spindle fiber formation and the cell division 10. Similarly antihypertensive alkaloids like ajmalicine and serpentine are found in the roots of these plants 11, 12. Apart form these activities this plant is also found to be anti-microbial where extracts of the plant is found to be effective against S. typhi, S. aureus and B. subtilis13, 14. Other than these numerous similar pharmaceutical activities such as antidiabetic, melanomas of breast and lung, Hodgkin’s and Non-Hodgkin’s lymphoma are also described for this plant15. The water extract of this plant is also known to be effective against bleeding, fever and rheumatisim 16. The anti-oxidant activity (anti-2, 2, 1-diphenyl-1-picrylhydrazyl) of this plant was also recently documented 17, 18. Extensive work has been carried out on the use of this plant against a wide range of diseases 17, 19, 20, 21, 22, 23, 24, 25, 26. Owing to very trace amount in C. roseus, has initiated widespread search for finding alternative solutions for cost effective and mass production of these drugs 7, 27, 28.
Plant cell and tissue culture technique is widely used method to enhance the accumulation of such therapeutic secondary metabolites in a wide range of plants 29. The cultured plant tissues have demonstrated much higher concentrations of alkaloids than the intact plants 30, 31, 32. The biotechnological approaches to manipulate and regulate the production of these alkaloids of C. roseus have been overviewed recently 7.
In the present study we are applying factors such as physical state, concentrations and types of nutrient salts (MS and B5), the level of sucrose, and combinations of plant growth regulators with the aim to create stress conditions similar to that experience by plant in the field as an inductive force for enhanced production of total alkaloids containing higher amount of anticancer vincristine and vinblastine.
Materials and Methods
Selection of Plants and Explants
The Catharanthus roseus L.were grown as seedlings in the GITAM campus as hedge plant. Axillary buds shoot tips, leaf segment and roots of six week old plants growing in the GITAM campus were collected for the culture establishment. These explants were used for callus induction following standard protocol 33. Most of these explants were not successfully established due to heavy contamination by endophytic fungi (Fusarium oxysporum) and bacteria 34. Since the leaf segments were only successful in culture establishment, further studies was confined with leaf segments as explants of choice.
Explants Preparation and Culture Establishment
The leaf explants were collected washed thoroughly under running tap water for 15 min. Surface disinfection was carried by treating with amild antiseptic solution (1% Savlon, GSK India) and 8-10 drops of Tween-20 for 5-8 min by continuous shaking. Further, surface sterilization was carried under aseptic environment over a Laminar Air Flow hood by treating first with 70% ethanol for 60 seconds followed by 0.05% HgCl2 for about 15 minutes with continuous stirring to ensure complete sterilization. Thereafter the explants were washed 3-4 times with autoclaved double distilled water to remove traces of HgCl2. The surface sterilized leaf explants were inoculated on the variously modified culture medium. The leaf explants were prepared either as circular disks with 1 cm diameter using a sterile cork borer or a squire segment of 1 cm2.
Culture Medium and Culture Conditions
Initially cultures were established following the standard protocols 33. At first normal strength Murashige and Skoog (MS) 35 medium containing 3 % (w/v) sucrose and different combinations of 4-D, Kinetin, 2 and NAA was for culture establishment. Later normal strength B5 culture medium 36 was modified variously for optimization. The leaf explants were treated continuously for 4-weeks in the presence of above growth regulators and each experiment was repeated three times. The leaf explants were also established on B5 medium following similar combinations of growth regulators along with 3% sucrose.
To study the effect of nutrient strength on growth and multiplication of callus, biomass and alkaloid yield three different types of nutrient media (half strength, full strength and one and half strength) was prepared for both MS and B5 in agar and in suspension along with standard sucrose and PGR combination. Callus and biomass obtained from both the medium were further used for alkaloid extraction and quantification.
The pH of the culture media was adjusted to 5.8 ± 0.2 prior to autoclaving (121°C, 15 min) and solidified with 1% agar (if required). The cultures were maintained at 25 ± 2 °C in an environmentally controlled air conditioned room. The culture racks were provided with a 16 h photoperiod under a photon flux density of 2,000-3,000 Lux, provided by fluorescent lamps.
Induction and Multiplication of Callus and Biomass
Induction of callus from leaf explants was initiated as per the standard protocol 33 with slight modifications. To test the effect of growth regulators treatments on callus induction different combinations permutations of 2, 4-D, Kinetin, NAA were used.
To study the combined effect of sucrose and various PGR combinations on growth and multiplication of callus and biomass and alkaloid yield three concentrations of sucrose (1%, 3%, 5%) were used in this study in normal strength of agar as well as in suspension for both MS and B5 formulations. Three different combinations of PGR’s used were (A) 0.1 mg/l of 2, 4-D + 0.5 mg/l of KIN + 1.0 mg/l of NAA (B) 0.5 mg/l of 2,4-D + 1.0 mg/l of KIN + 2.0 mg/l of NAA and (C) 1.0 mg/l of 2,4-D + 2.0 mg/l of KIN + 4.0 mg/l of NAA.). Callus and biomass obtained from both the medium were further used for alkaloid extraction and quantifications.
The biomass doubling time was calculated by harvesting the suspension culture from 3 conical flasks after every 2-day interval filtered on a pre-weighed filter paper. The suspension cultures were maintained by continuous shaking at 150 RPM in a shaker incubator for 12-14 days. The suspension cultures were established and maintained for 2 cycles and sub-cultured every 12-14 days for the cell biomass harvesting and alkaloid yield. The cell biomass was harvested after 14 - days (2– weeks) by filtration on a pre-weighed filter paper (Whatman No.1).
Sample Size
For callus culture 100ml of medium was modified for each treatment and dispensed equally in 2 culture boxes. Each culture box was inoculated with 4 - leaf segments for callus induction or 4 – spots of 100 -150 mg of callus for sub-culture and multiplications. Hence, induction and proliferation of callus is represented by a sample size of 8 per 100ml culture medium per treatment. Callus cultures were maintained by sub-culturing every 4-weeks cycle.
The friable callus at the end of second subculture was used to establish suspension culture. In suspension culture each treatment was modified for 100ml of medium and dispensed 25ml each in to 4 - conical flasks of 150ml capacity. Each 25ml of suspension medium was inoculated with 100 – 150 mg of friable callus (4 – weeks old) from agar medium or 5ml of 2 – weeks old suspension culture for biomass production. Hence, sub-culture, proliferation and biomass production in suspension culture was represented by a sample size of 4 per 100ml medium per treatment.
The growth and development was measured by pooling callus from 2 culture boxes for 100ml medium, expressed in g/l and considered as first replicate. Similarly, cell biomass from 100ml of suspension medium was pooled, expressed in g/l unit and considered as first replicate. These experiments on each treatment were repeated thrice and the results represented as mean ± standard deviations.
Growth Measurement
Callus Growth
The callus grown on agar medium was harvested after 4-weeks of sub-culture. The cell biomass from suspension culture was harvested every 2- weeks of culture/sub-culture. Callus grown in each of the culture tube or culture boxes were weighed separately.
The laboratory filter paper cut to the size of inner Petri-plate pair and dried in oven at 60°C for 2hrs to make the papers completely moisture free, cooled down to room temperature before weighing. To calculate callus fresh weight, the initial weight of dried filter paper was taken followed by weight of filter paper along with callus and represented as:
Callus Fresh Wt = (Weight of filter paper and the callus – Initial weight of dried filter paper)
To calculate dry weight, the callus along with the filter was dried by initially at room temperature for 2 - 4 days. The callus along with filter paper was further incubated at 40°C in dry heat oven for 3 – 4 hours to remove the traces of moisture before weighing for dry weight. Precaution was maintained to avoid moisture exposure of petri-pales with dried callus and the filter paper. The dried callus along with the filter paper was weighed and represented as:
Callus Dry Wt = (Weight of dried callus along with filter paper – Initial weight of dried filter paper)
Cell Biomass Growth
The cell biomass growth was calculated by harvesting the suspension culture from all 4 conical flasks of a treatment after every 2-days interval onto a pre-weighed filter paper. Filter papers (Whatman No.1) were dried in oven at 60°C for 2hrs to make the papers completely moisture free, cooled down to room temperature before weighing. One of the dried filter paper discs was used to filter simple distilled water followed by keeping over three layer of blotting paper towel for 1 hr with 2-3 changes to absorb moisture. The weight of moisture free filter paper was taken.
The cell biomass were harvested by pooled filtration from 100ml medium for each treatment using the pre-weighed filter paper and kept in the petri-plates over 3 layers of blotting papers towel for absorbing of moisture by changing 2-3 times with fresh towels over a period of 1 hr. To calculate cell biomass fresh weight, the weight of moisture free filter paper along with cell biomass was taken and represented as:
Biomass Fresh Wt = (Weight of moisture free filter paper and the cell biomass – Initial weight of moisture free filter paper)
The cell biomass was allowed to dry at room temperature for 2-3 days by shifting on a fresh blotting papers towel every day. Finally cell biomass was dried in oven at 40°C for 2-3 hr to remove the remaining moisture before weighing for dry weight. Precaution was maintained to avoid moisture exposure of petri-pales with cell biomass. The dried cell biomass was calculated as:
Biomass Dry Wt = (Weight of the dried biomass with filter paper – Weight of the dried filter paper)
Extraction of Total Alkaloids
About 20mg of dried callus or cell biomass was ground with 10ml of methanol in a mortar pestle, and the whole mixture kept overnight in 100ml conical flasks in a rotary shaker at 25 – 50 rpm for proper mixing of the solvent. The total mixture was then centrifuged at 3000 RPM for 10 minutes and about 9.0 ml of supernatant collected. The supernatant was re-centrifuged 2-3 times until a clear supernatant was obtained. Finally 7-8ml of supernatant was collected in 25ml beakers and kept for drying at room temperature. After complete evaporation of solvent (overnight) the left over content was re-dissolved in 2ml methanol (by mixing for 1hr covered with Petri-plates) and stored in 2.0ml micro-centrifuge vials for further investigations.
Alkaloid Quantifications
Preparation of Standard curve for total Alkaloid Quantification
The calibration curve was prepared with Bismuth nitrate pentahydrate (Bi(NO3)3.5H2O) stock solution. Bismuth nitrate stock solution was made by dissolving 10mg Bismuth nitrate and diluted with DDW (10mg of Bi(NO3)3.5H2O + 5ml of Conc. HNO3 + 95ml of DDW = 100ml of Bi(NO3)3.5H2O). Series dilutions of the stock solution were made by pipetting out 0, 1, 2, 3, 4, 5, 6, 7, 8, and 9 mL stock solution into separate 10mL standard flasks and diluted to volume with double distilled water.
A 1mL amount of this solution was taken, and 5 mL thiourea solution (3%) was added to it. The absorbance value of the light yellow solution was measured at 435 (430) nm against the blank containing nitric acid and thiourea. The OD value against each of the concentration is presented in Figure 1.
Figure 1.The calibration curve of Bismuth nitrate pentahydrate (Bi (NO3)3.5H2O) for the quantification of total alkaloids.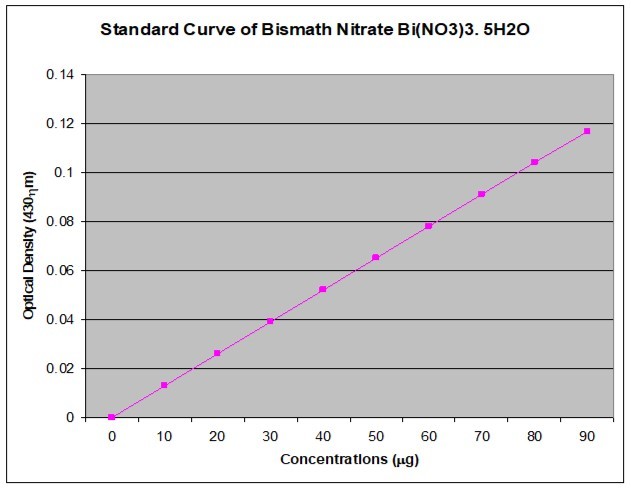
Quantitative Estimation of Total Alkaloids
The total alkaloid was estimated following the modification of protocol 33, 37. A 1 mL of methanolic extract was taken and the pH brought down at 2–2.5 (pH paper) with dilute HCl. A 400µL amount of Dragendorff’s (DR) reagent (prepared by mixing equal volume of i. solution of 0.8g bismuth nitrate pentahydrate in 40 ml distilled water and 10 ml glacial acetic acid, and ii. solution of 8.0g potassium iodide in 20 ml distilled water used as stock solution) was added to it, and the precipitate formed was centrifuged for 10mins at 5000rpm. The centrifugate was checked for complete precipitation by adding additional 400µL of DR solution. The centrifugate was discarded completely and the precipitate was further washed twice with methanol. The filtrate was discarded and the residue was then treated with 400µL disodium sulfide (1% in DDW) solution. The resulting brownish black precipitate was then centrifuged for 10mins at 5000rpm. Completion of reaction was checked by adding 2 drops of disodium sulfide and centrifuged again. The residue was dissolved in 400µL concentrated nitric acid by gentle warming. This solution was added with 1.6 ml of DDW to get a final mixture of 2ml. A 1 mL volume of this mixture was mixed with 5 mL thiourea solution (3% in DDW). The absorbance was measured at 435 (430) nm against the blank containing nitric acid and thiourea. The amount of bismuth present in the solution was calculated by multiplying the absorbance values with the factor taking suitable dilution factor into consideration. The factor was calculated from standard graph shown in Figure 1 as a constant value for different concentrations.
Factor = Concentration / absorbance at 430nm
or
Concentration = Factor x Absorbance at 430nm
The alkaloid content was represented as mg/g dry weight of callus or the cell biomass and calculated as under:
Alkaloid content (mg/g dwt): {(Concentrations ((µg) / 10mg Dry weight} x 1000
Alkaloid Production
It is defined as the alkaloid content per litre of the culture establishment. It is calculated as:
Alkaloid Production (mg/l) = Dry wt (g/l) x Alkaloid content (mg/g)
Alkaloid Productivity
The rate of production of alkaloid per litre per day is the productivity of system (treatment). It is calculated as
Alkaloid Productivity (mg/l/day) = {Alkaloid Production (mg/l) / no. of days the product is harvested}
Alkaloid Yield
The yield of the alkaloid per treatment is represented as the percentage of alkaloid per gram dry weight of the callus or the cell biomass produced. It is calculated as
Alkaloid Yield (% dwt) = {Alkaloid Content (mg/g) / 1000} x 100
Statistical Analysis
The mean of three replicates of experiments, standard deviation, monovariate and multivariate analysis of variance (ANOVA and MANOVA) of results were performed by using SPSS 15 package for Window (SPSS Inc., USA) in the present study.
Result and Discussion
Callus Induction, Growth and Development
Both axillary buds and shoot tip explants were found difficult for successful establishment of cultures due to high contamination (100%) by endophytic fungus Fusarium oxysporum34. Induction of callus from leaf explants was initiated as per the protocol 33 with different combinations permutations of 2, 4-D, Kinetin, NAA each at a concentrations of 0.0, 0.5, 1.0, 2.0 and 4.0 mg/l as randamized design in normal strength MS agar medium with 3% sucrose. The leaf segments used for culture establishment have shown 20-30% contaminations (mostly bacterial) during 3-4 weeks of culture initiation. About 88-100% of the leaf segments (1 cm2) have shown the callus formation within a week of culture initiation. The callus mass continued to grow slowly and ceased growing beyond 4- weeks of culture initiation. There was very fast growth of callus when sub-cultured on to fresh medium. The result of callus development from leaf explants and growth in the first and second sub-culture is presented in Figure 2. Induction and development of callus from leaf explants as well as their growth in the first and second sub-culture was found best in a combination of 0.5 mg/l of 2,4-D + 1.0 mg/l of KIN + 2.0 mg/l of NAA in 3% (w/v) sucrose containing MS normal strength agar solidified medium. . Callus sub-cultured for more than 2 cycles (4-weekd each) were prone to bacterial contaminations (20-30%) and became more friable with diverse cyto-differentiation and irregular morphology. While those maintained for longer than 4 weeks onto the same culture media develop into more compact, solid calli. Therefore, multiplication of callus was performed only for two subcultures cycle to maintain the uniformity in morphological characteristics and the alkaloid content. As a consequence fresh callus induction was performed from leaf explants for every 12-16 weeks. Both MS and B5 medium supplemented with various growth regulators (NAA, Kinetin, BAP, 2, 4-D etc.) and other growth supplements were reported to induce callus and biomass containing these alkaloids is enhanced 29, 38, 39, 40, 41.
Figure 2.Interactive effects of 2, 4-D, Kinetin, NAA on induction and multiplication of callus growth and development*.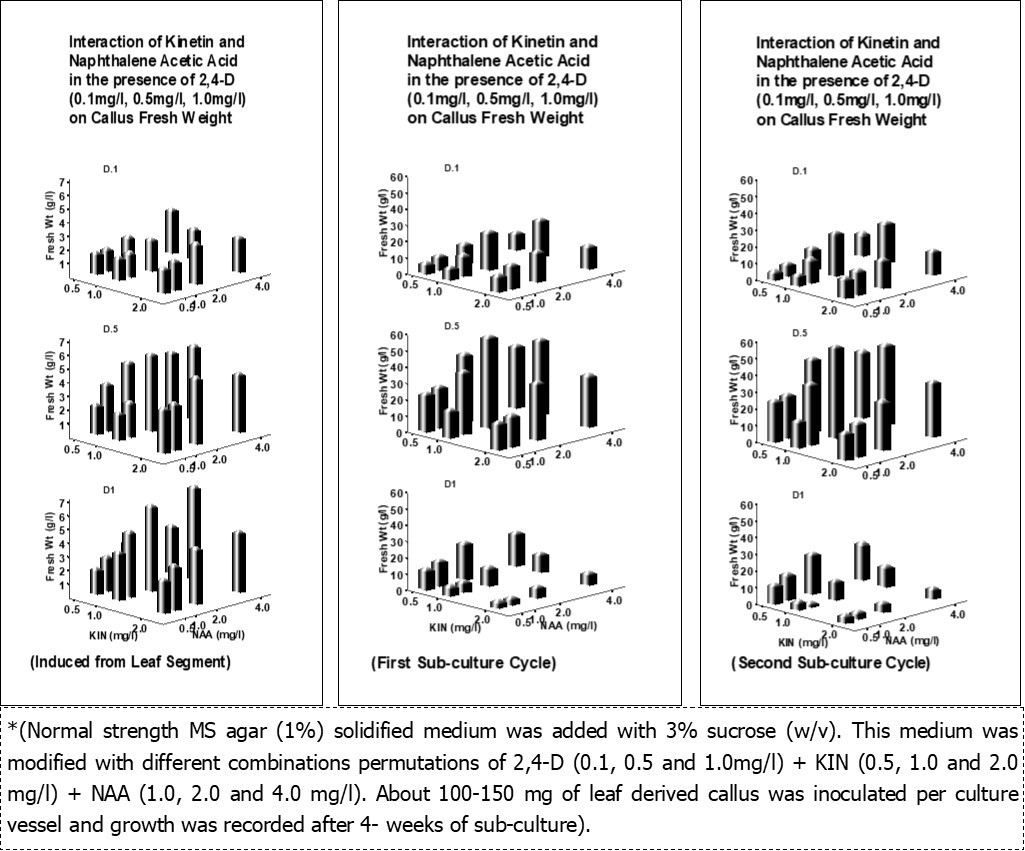
Growth Rate of Cell Biomass
The growth rate in suspension culture was calculated by harvesting cell biomass after every 2-day interval over a period of 1 month. The fresh weight of biomass growth calculated for 4-flasks was represented as mg/100ml suspension culture medium as shown in Figure 3. The cell biomass in suspension culture followed a sigmoid pattern with 2-3 days of lag period followed by exponential growth during 3-9 days of culture initiation. The response plateau formed during 10-14 days of culture beyond which there was as a stationary without any change in growth of cell biomass between 14-28 days of culture.
Figure 3.Growth rate of cell biomass in 2nd and 3rd subculture cycle*.
Effects of Strength and Physical State of Medium
All the observable response of fresh wt., dry wt., alkaloid content, production, productivity and the yield showed different pattern in two different physical state (agar and suspension) of the medium irrespective of MS or the B5 nutrient formulation (Table 1, Table 2, Table 3, Table 4). The B5 nutrient formulation in agar solidified condition showed higher response of all the parameter compared to the MS agar medium irrespective of their strength (half, normal or one and half) (Table 1 and Table 3). Strength of medium (half, normal or one and half) in both the nutrient (B5 or MS) formulations under agar solidified conditions exerted significant effect with the maximum response of all the observable parameters in full strength medium and the least response in half strength medium (Table 1 and Table 3). Enhancing the nutrient levels to one and half strength did not improve any of the observable response.
Table 1. Effect of Nutrient Strength on Growth and Multiplication of Callus and Alkaloid in MS Agar Medium*.| Sl. No. | Media Strength | Callus Fresh wt | Callus Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | ||
| 1 | 0.50 | 30.400 ± 4.331 | 3.042 ± 0.373 | 1.785 ± 0.147 | 5.396 ± 0.293 | 0.193 ± 0.011 | 0.179 ± 0.015 |
| 2 | 1.00 | 50.000 ± 2.425 | 5.230 ± 0.120 | 2.411 ± 0.262 | 12.595 ± 1.227 | 0.450 ± 0.044 | 0.241 ± 0.026 |
| 3 | 1.50 | 49.400 ± 2.905 | 5.108 ± 0.273 | 2.453 ± 0.115 | 12.545 ± 1.195 | 0.448 ± 0.043 | 0.245 ± 0.012 |
| F(2, 6) | 33.805 | 59.755 | 12.128 | 51.146 | 50.590 | 12.133 | |
| P<0.05 | 0.001 | 0.000 | 0.008 | .000 | 0.000 | 0.008 | |
| Sl. No. | Media Strength | Biomass Fresh wt | Biomass Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | ||
| 1 | 0.50 | 10.200 ± 0.144 | 1.153 ± 0.119 | 4.793 ± 0.311 | 5.546 ± 0.902 | 0.396 ± 0.064 | 0.479 ± 0.031 |
| 2 | 1.00 | 19.400 ± 0.262 | 2.070 ± 0.108 | 6.021 ± 0.351 | 12.451 ± 0.627 | 0.889 ± 0.045 | 0.602 ± 0.035 |
| 3 | 1.50 | 19.480 ± 0.560 | 2.010 ± 0.075 | 5.844 ± 0.231 | 11.761 ± 0.904 | 0.840 ± 0.065 | 0.584 ± 0.024 |
| F(2, 6) | 635.286 | 75.216 | 14.548 | 64.323 | 64.149 | 14.351 | |
| P<0.05 | 0.000 | 0.000 | 0.005 | .000 | 0.000 | 0.005 | |
| Sl. No. | Media Strength | Callus Fresh wt | Callus Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | ||
| 1 | 0.50 | 33.267 ± 2.053 | 3.242 ± 0.066 | 2.140 ± 0.185 | 6.930 ± 0.465 | 0.247 ± 0.016 | 0.214 ± 0.019 |
| 2 | 1.00 | 52.267 ± 2.641 | 5.450 ± 0.313 | 2.709 ± 0.057 | 14.769 ± 1.040 | 0.527 ± 0.037 | 0.271 ± 0.006 |
| 3 | 1.50 | 53.267 ± 1.501 | 5.521 ± 0.154 | 2.507 ± 0.097 | 13.838 ± 0.632 | 0.494 ± 0.023 | 0.251 ± 0.009 |
| F(2,6) | 85.045 | 120.057 | 16.017 | 97.465 | 99.465 | 15.934 | |
| P<0.05 | 0.000 | 0.000 | 0.004 | .000 | 0.000 | 0.004 | |
| Sl. No. | Media Strength | Biomass Fresh wt | Biomass Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | ||
| 1 | 0.50 | 10.560 ± 0.655 | 1.223 ± 0.060 | 5.101 ± 0.424 | 6.225 ± 0.378 | 0.445 ± 0.027 | 0.510 ± 0.043 |
| 2 | 1.00 | 20.307 ± 0.361 | 2.129 ± 0.079 | 6.203 ± 0.178 | 13.214 ± 0.825 | 0.944 ± 0.058 | 0.621 ± 0.018 |
| 3 | 1.50 | 20.253 ± 0.599 | 2.165 ± 0.075 | 5.970 ± 0.325 | 12.938 ± 1.094 | 0.924 ± 0.078 | 0.597 ± 0.032 |
| F(2, 6) | 308.481 | 167.032 | 9.600 | 69.794 | 70.130 | 9.578 | |
| P<0.05 | 0.000 | 0.000 | 0.013 | .000 | 0.000 | 0.014 | |
| PGR Comb. | Sucrose Conc. | Callus Fresh wt | Callus Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| % | g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | |
| A | 1 | 7.393 ± 0.303 | 1.887 ± 0.061 | 1.783 ± 0.178 | 3.371 ± 0.440 | 0.120 ± 0.016 | 0.178 ± 0.018 |
| A | 3 | 38.933 ± 2.403 | 4.230 ± 0.083 | 2.709 ± 0.057 | 11.455 ± 0.165 | 0.409 ± 0.006 | 0.271 ± 0.006 |
| A | 5 | 42.067 ± 1.206 | 4.301 ± 0.120 | 2.507 ± 0.097 | 10.778 ± 0.445 | 0.385 ± 0.016 | 0.251 ± 0.009 |
| B | 1 | 33.067 ± 2.194 | 3.375 ± 0.174 | 2.079 ± 0.174 | 6.999 ± 0.379 | 0.250 ± 0.014 | 0.208 ± 0.018 |
| B | 3 | 49.133 ± 2.845 | 5.215 ± 0.294 | 3.101 ± 0.077 | 16.181 ± 1.249 | 0.578 ± 0.044 | 0.310 ± 0.008 |
| B | 5 | 45.067 ± 3.101 | 4.297 ± 0.163 | 2.873 ± 0.077 | 12.352 ± 0.785 | 0.441 ± 0.028 | 0.287 ± 0.008 |
| C | 1 | 23.800 ± 1.778 | 2.359 ± 0.134 | 2.109 ± 0.132 | 4.980 ± 0.520 | 0.178 ± 0.019 | 0.211 ± 0.013 |
| C | 3 | 47.400 ± 1.114 | 4.863 ± 0.264 | 2.829 ± 0.065 | 13.746 ± 0.432 | 0.491 ± 0.016 | 0.283 ± 0.006 |
| C | 5 | 48.533 ± 0.702 | 4.374 ± 0.133 | 2.395 ± 0.157 | 10.475 ± 0.699 | 0.374 ± 0.025 | 0.240 ± 0.016 |
| Source | Dependent Variable | Type III Sum of Squares | df | Mean Square | F | Sig. |
| Corrected Model | Biomass Fresh wt | 4645.515(a) | 8 | 580.689 | 150.473 | .000 |
| Biomass Dry wt | 30.024(b) | 8 | 3.753 | 123.527 | .000 | |
| Alkaloid Content | 4.520(c) | 8 | .565 | 38.367 | .000 | |
| Alkaloid Production | 416.568(d) | 8 | 52.071 | 127.166 | .000 | |
| Alkaloid Productivity | .532(d) | 8 | .066 | 127.846 | .000 | |
| Alkaloid Yield | .045(c) | 8 | .006 | 38.416 | .000 | |
| Intercept | Biomass Fresh wt | 37496.229 | 1 | 37496.229 | 9716.361 | .000 |
| Biomass Dry wt | 406.019 | 1 | 406.019 | 13363.633 | .000 | |
| Alkaloid Content | 167.029 | 1 | 167.029 | 11341.884 | .000 | |
| Alkaloid Production | 2720.338 | 1 | 2720.338 | 6643.545 | .000 | |
| Alkaloid Productivity | 3.468 | 1 | 3.468 | 6673.151 | .000 | |
| Alkaloid Yield | 1.670 | 1 | 1.670 | 11357.991 | .000 | |
| PGR Comb. | Biomass Fresh wt | 850.028 | 2 | 425.014 | 110.133 | .000 |
| Biomass Dry wt | 3.053 | 2 | 1.526 | 50.236 | .000 | |
| Alkaloid Content | .580 | 2 | .290 | 19.701 | .000 | |
| Alkaloid Production | 50.534 | 2 | 25.267 | 61.707 | .000 | |
| Alkaloid Productivity | .064 | 2 | .032 | 61.694 | .000 | |
| Alkaloid Yield | .006 | 2 | .003 | 19.817 | .000 | |
| Sucrose Conc. | Biomass Fresh wt | 3389.780 | 2 | 1694.890 | 439.195 | .000 |
| Biomass Dry wt | 25.046 | 2 | 12.523 | 412.173 | .000 | |
| Alkaloid Content | 3.708 | 2 | 1.854 | 125.898 | .000 | |
| Alkaloid Production | 357.128 | 2 | 178.564 | 436.084 | .000 | |
| Alkaloid Productivity | .456 | 2 | .228 | 438.790 | .000 | |
| Alkaloid Yield | .037 | 2 | .019 | 125.986 | .000 | |
| PGR Comb. * Sucrose Conc. | Biomass Fresh wt | 405.707 | 4 | 101.427 | 26.283 | .000 |
| Biomass Dry wt | 1.926 | 4 | .482 | 15.850 | .000 | |
| Alkaloid Content | .232 | 4 | .058 | 3.934 | .018 | |
| Alkaloid Production | 8.906 | 4 | 2.226 | 5.437 | .005 | |
| Alkaloid Productivity | .011 | 4 | .003 | 5.450 | .005 | |
| Alkaloid Yield | .002 | 4 | .001 | 3.931 | .018 | |
| Error | Biomass Fresh wt | 69.463 | 18 | 3.859 | ||
| Biomass Dry wt | .547 | 18 | .030 | |||
| Alkaloid Content | .265 | 18 | .015 | |||
| Alkaloid Production | 7.370 | 18 | .409 | |||
| Alkaloid Productivity | .009 | 18 | .001 | |||
| Alkaloid Yield | .003 | 18 | .000 |
| PGRComb. | Sucrose Conc. | Biomass Fresh wt | Biomass Dry wt | Alkaloid Content | Alkaloid Production | Alkaloid Productivity | Alkaloid Yield |
| % | g/l | g/l | mg/g dwt | mg/l | mg/l/d | % dwt | |
| A | 1 | 11.69 ± 0.69 | 1.29 ± 0.08 | 4.49 ± 0.27 | 5.78 ± 0.43 | 0.41 ± 0.03 | 0.45 ± 0.03 |
| A | 3 | 14.85 ±0.65 | 1.73 ± 0.05 | 5.82 ± 0.04 | 10.05 ± 0.28 | 0.72 ± 0.02 | 0.58 ± 0.00 |
| A | 5 | 15.80 ± 0.48 | 1.61 ± 0.04 | 5.46 ± 0.08 | 8.77 ± 0.08 | 0.63 ± 0.01 | 0.55 ± 0.01 |
| B | 1 | 17.68 ± 0.14 | 2.01 ± 0.04 | 5.28 ± 0.19 | 10.61 ± 0.53 | 0.76 ± 0.04 | 0.53 ± 0.02 |
| B | 3 | 18.41 ± 0.32 | 2.12 ± 0.13 | 6.00 ± 0.28 | 12.68 ± 0.91 | 0.91 ± 0.07 | 0.60 ± 0.03 |
| B | 5 | 18.35 ± 0.12 | 2.02 ± 0.15 | 5.67 ± 0.31 | 11.47 ± 0.98 | 0.82 ± 0.07 | 0.57 ± 0.03 |
| C | 1 | 13.63 ± 0.62 | 1.16 ± 0.08 | 4.61 ± 0.20 | 5.37 ± 0.56 | 0.38 ± 0.04 | 0.46 ± 0.02 |
| C | 3 | 16.80 ± 0.74 | 1.90 ± 0.08 | 6.33 ± 0.32 | 12.01 ± 0.87 | 0.86 ± 0.06 | 0.63 ± 0.03 |
| C | 5 | 16.91 ± 0.80 | 2.00 ± 0.06 | 5.15 ± 0.20 | 10.32 ± 0.21 | 0.74 ± 0.02 | 0.52 ± 0.02 |
| Source | Dependent Variable | Type III Sum of Squares | df | Mean Square | F | Sig. |
| Corrected Model | Biomass Fresh wt | 123.446(a) | 8 | 15.431 | 48.654 | .000 |
| Biomass Dry wt | 2.828(b) | 8 | .354 | 47.076 | .000 | |
| Alkaloid Content | 8.996(c) | 8 | 1.124 | 21.229 | .000 | |
| Alkaloid Production | 161.241(d) | 8 | 20.155 | 52.594 | .000 | |
| Alkaloid Productivity | .823(e) | 8 | .103 | 52.218 | .000 | |
| Alkaloid Yield | .090(f) | 8 | .011 | 21.098 | .000 | |
| Intercept | Biomass Fresh wt | 6923.525 | 1 | 6923.525 | 21830.060 | .000 |
| Biomass Dry wt | 83.544 | 1 | 83.544 | 11124.273 | .000 | |
| Alkaloid Content | 794.009 | 1 | 794.009 | 14989.631 | .000 | |
| Alkaloid Production | 2525.669 | 1 | 2525.669 | 6590.620 | .000 | |
| Alkaloid Productivity | 12.885 | 1 | 12.885 | 6538.928 | .000 | |
| Alkaloid Yield | 7.941 | 1 | 7.941 | 14892.169 | .000 | |
| PGR Comb. | Biomass Fresh wt | 73.873 | 2 | 36.937 | 116.462 | .000 |
| Biomass Dry wt | 1.238 | 2 | .619 | 82.391 | .000 | |
| Alkaloid Content | .739 | 2 | .369 | 6.974 | .006 | |
| Alkaloid Production | 54.327 | 2 | 27.164 | 70.883 | .000 | |
| Alkaloid Productivity | .277 | 2 | .139 | 70.388 | .000 | |
| Alkaloid Yield | .007 | 2 | .004 | 6.971 | .006 | |
| Sucrose Conc. | Biomass Fresh wt | 38.589 | 2 | 19.295 | 60.836 | .000 |
| Biomass Dry wt | 1.011 | 2 | .506 | 67.312 | .000 | |
| Alkaloid Content | 7.099 | 2 | 3.550 | 67.009 | .000 | |
| Alkaloid Production | 87.883 | 2 | 43.941 | 114.663 | .000 | |
| Alkaloid Productivity | .449 | 2 | .224 | 113.827 | .000 | |
| Alkaloid Yield | .071 | 2 | .035 | 66.515 | .000 | |
| PGR Comb. * Sucrose Conc. | Biomass Fresh wt | 10.984 | 4 | 2.746 | 8.658 | .000 |
In Podophyllum peltatum tissue cultures Kadkade 42, 43 showed that full strength MS medium was best suited for callus and podophyllotoxins production. Similar results were also observed by Drewes & Staden 44 for solasodine production in Solanum mauritianum. Likewise Rosli et al. 45 showed that 9-methoxycanthin-6-one production was enhanced in Eurycomalongifolia callus cultures. The concentration of basal medium was also reported to contribute towards the variation of solasodine production in callus cultures of Solanum aviculare46. Similar findings were observed where along with auxins normal strength MS medium was found to be best for callus production of C. roseus 47.
Suspension Medium
The fresh and dry weight of the cell biomass was reduced 2-3 times in suspension condition when compared to agar solidified medium of both the nutrient (B5 and MS) formulation (Table 2 and Table 4). However, the alkaloid content, productivity and the yield were enhanced almost 2-3 time in suspension conditions as compared to agar solidified medium in their respective formulations and strength (Table 2 and Table 4). The total alkaloid production remained unchanged irrespective of the strength and the physical state of both (B5 and MS) the nutrient formulation.
Strength of the medium played a significant role with maximum response of all the observable parameters in normal strength and least response in half strength suspension culture (Table 2 and Table 4). Hence it was inferred that normal strength MS medium along with standard sucrose and PGR combinations is the best combination for biomass and alkaloid yield in suspension culture.
The culture production conditions of Egyptian henbane had shown that full strength MS medium as the best suited for callus and metabolites production 48. Similar results were also observed 49, 50, 51 for alkaloid production in Holarrhenaantidysenterica to study the effect of biochemical engineering on secondary metabolites derived from plant source by implementation of biochemical engineering production using cell suspension culture. MS suspension culture at normal strength proved to be the best nutrient source for biomass and alkaloid production 52 while studying the production of some important secondary metabolites from medicinal plants by plant tissue cultures.
These findings corroborated with the findings where cytokinin in B5 media was found to very helpful in increasing the total alkaloid content in Catahranthus roseus 53, 54. Hence, it was inferred that both normal strength MS and B5 suspension culture along with standard sucrose and PGR combinations as the best combination with better response of biomass and alkaloid yield in B5 suspension culture. Similar finding has proved that Catharanthus roseus biomass when transferred to B5 suspension medium showed extensive alkaloid production when used as immobilized cells 55, 56.
Effect of Sucrose and PGR’s
MS Agar Medium
The result of different concentrations of sucrose (1%, 3% and 5%) along with three variable combinations of PGR’s in MS agar medium is shown in Table 5. Multivariate analysis of variance (MANOVA) was performed and presented in Table 5(a). Both sucrose and PGR’s significantly affect all observable parameters when analyzed alone irrespective of other factor as well as interact significantly when analyzed in combined form Table 5(a). The lower concentrations of both sucrose and PGR’s were found significantly less effective than their higher concentrations. Sucrose at 3% concentrations along with PGR’s combinations B (Table 5 footnote) were sufficient for maximum responses of callus fresh wt, dry wt, alkaloid content, production, productivity and the yield in MS agar medium. A 5% sucrose containing MS agar medium in all the PGR’s combinations were found to induce improved responses but slightly lesser than that shown by 3% sucrose interacting in a significant manner.
These results confirm that induction, growth, multiplications and alkaloid production from callus culture of Catharanthus roseus depends strongly on the concentrations of both sucrose as the carbon source and PGR’s. The favorable effect of sucrose in this study can be explained by the fact that sucrose gets easily assimilated by the cells of C. roseus. Similar results were reported by Petersen et al. 57 where they showed that sucrose at 3% concentrations had improved significantly the induction, maintenance and plant regeneration from embryogenic callus of Miscanthus x ogiformis Honda ‘Giganteus’. It was also observed that sucrose at 3% in basal MS medium was best for callus development for Solanum nigrum 58. Earlier Kadkade 43 showed sucrose as the best carbon source for the production of podophyllotoxin in callus culture of Prodophyllumpeltatum while glucose, fructose and galactose were less effective. Bondarev et al. 59 optimzed accumulation of steviol glycoside from developing Stevia rebaudiana shoots cultivated in the roller bioreactor using nutrient medium containing 3% sucrose. Enhanced alkaloid content was from callus and cell biomass of C. roseus with maximum production in the presence of 3% glucose followed lactose and maltose each at 3% level respectively along with standard sucrose (3%) in the medium 60.
MS Suspension Medium
The effect of different combination permutations of sucrose and PGR’s in MS suspension medium on biomass and alkaloid production is presented in Table 6. Multivariate analysis of variance (MANOVA) was performed for these results and presented in Table 6(a). Both sucrose and PGR’s in MS suspension medium interacted significantly affecting all the observable parameters irrespective of whether analyzed alone or in different combinations permutations Table 6(a). In MS suspension medium fresh wt and dry wt of the cell biomass were significantly (2-3 times) less compared to agar medium. Whereas alkaloid content, productivity and the yield were 2-3 times higher than that produced in MS agar medium. The alkaloid production (in mg/l) remained unaffected irrespective to the level of sucrose or the PGR combinations in suspension medium (Table 6). The lower concentrations of both sucrose and PGR’s were found least responsive than the higher concentration. All the responses were at best in the presence of 3% sucrose along with PGR’s combination B (Table 6 footnote). The 5% sucrose in MS suspension medium showed enhanced responses but slightly lesser than that observed in 3% sucrose irrespective of PGR’s combinations but interacted significantly.
Amongst the two physical states of media, MS nutrient salts in suspension condition produced enhanced amount of alkaloid content, productivity and the yield despite the compromised fresh and dry wt of biomass as compared to agar solidified medium. Additionally, cell biomass culture cycle in suspension medium required just half the time (14-days) compared to callus multiplication cycle (28-days) in agar solidified medium (see footnotes of Table 5,Table 6). Hence, suspension medium were found more efficient for enhancing the alkaloid content, productivity and the yield compared to agar solidified medium.
Sucrose at 3% was beneficial in the production of ajamalicine and catharanthine from immobilized cells of C. roseus using a conditioned medium 61. However, sucrose ranging from 2-6% increases the production of arbutin from suspension cultures of C. roseus by glucosylation of exogenous hydroquinone 62. Maximum accumulation of betacyanin in suspension culture of Phytolaccaamericanawas enhanced by increasing the cell number in the presence of 88mM sucrose and by fresh weight in 175mM sucrose containing medium 63. Additionally, sugar type and the osmotic potential of the culture medium affected the somatic embryogenesis in Euonymus europaeus64. Fragaria ananassacallus when grown in dark in MS medium in the presence of sucrose at 3% showed enhanced production of anthocyanin content 65.
Conclusion
In the present study we concluded that there exist a strong interaction of PGR’s and sucrose concentrations for the induction, growth and multiplication of leaf derived callus in both MS agar and suspension medium. Further, the physical state of the medium (agar solidified and liquid suspension) played an important role on all the observable parameter of fresh wt and dry wt as well as alkaloid content, production, productivity and yield. The standard culture conditions induced considerable amount of variations in total alkaloid production from callus grown on agar medium and the cell biomass in suspension of Catharanthus roseus. Different factors such as concentrations of nutrient salts and sucrose used as carbon source along with the plant growth regulators tested in two media formulations (MS and B5 salts) significantly effected all the parameters of fresh wt, dry wt, alkaloid content, production, productivity and yield irrespective of whether analyzed alone or in combinations. The yield of total alkaloid was optimized for enhanced production in different combinations permutations of nutrient salts, sucrose and plant growth regulators. There was considerably enhanced amount (5.67 mg/g dwt) of total alkaloid yield in the present study similar to that observed in other reports 61, 62, 63, 64, 65. The obtained results need to be exploited further for separation and purification of different indole alkaloids to test their pharmacological activities.
Acknowledgement
The financial support provided by University Grant Commission, New Delhi for the major research project (F.: 42-207/2013 (SR) for the period 1.4.2013-31.3.2017) to the corresponding author is gratefully acknowledged. All the authors are grateful to Dr. K. V. Chaitanya for his critical review and editing of the manuscript.
Contribution of Authors
All the experiments were executed and performed by Mr. Malay Ranjan Mishra. The second author Dr. Rajesh K. Srivastava constantly reviewed the experiments and results of various experiments. The experiment design and planning for execution of entire study was conducted by the corresponding author Dr. Nasim Akhtar to achieve the objective of the major research project sanctioned to him by the funding agency University Grant Commission, New Delhi (F.: 42-207/2013 (SR) for the period 1.4.2013-31.3.2017). All the authors declared that there is no conflict with regards to any part of the manuscript.
References
- 1.Pan Q, Mustafa N R, Tang K, Choi Y H, Verpoorte R. (2016) Monoterpenoid indole alkaloids biosynthesis and its regulation inCatharanthusroseusa literature review from genes to metabolites. Phytochemistry reviews. 15(2), 221-250.
- 2.Zhu J, Wang M, Wen W, Yu R. (2015) Biosynthesis and regulation of terpenoid indole alkaloids inCatharanthusroseus. Pharmacognosy reviews. 9(17), 24-28.
- 3.Ishikura M, Abe T, Choshi T, Hibino S. (2015) Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Natural product reports. 32(10), 1389-1471.
- 4.Verpoorte R, Heijden R Van der, Schripsema J, JHC Hoge, Ten Hoopen HJG. (1993) Plant cell biotechnology for the production of alkaloids: present status and prospects. , J Nat Prod 56, 186-207.
- 5.Almagro L, Fernández-Pérez F, Pedreño M A. (2015) Indole alkaloids fromCatharanthusroseusbioproduction and their effect on human health. , Molecules 20(2), 2973-3000.
- 6.Hughes E H, Shanks J V. (2002) Metabolic engineering of plants for alkaloid production. , Metabolic Engineering 4(1), 41-48.
- 7.Naeem M, Aftab T, MMA Khan.(2017)Catharanthusroseus: Current Research and Future Prospects. , AG, Gewerbestrasse, Switzerland,pp 441.
- 8.Shabbir A, Ali A, Sadiq Y, Jaleel H, Ahmad B. (2017) Unraveling the Cumulative Effect of Soil-Applied Radiation-Processed Sodium Alginate and. Polyacrylamide on Growth Attributes, Physiological Activities, and Alkaloids Production in Periwinkle [Catharanthusroseus , Cham 365-381.
- 9.Verpoorte R, Heijden R Van der, Moreno P R. (1997) Biosynthesis of terpenoid indole alkaloids inCatharanthusroseuscells. In The Alkaloids: Chemistry and Pharmacology, Academic Pres 49, 221-299.
- 10.Creasey W A. (1979) The vinca alkaloids. In:Hahn,F.E.et al(eds)Antibiotics.5.Springer-Verlag , New York 414-438.
- 11.Lemmens R H, Bunyapraphatsara M J, Padua de LSN. (1999) . Plant Resources of South-East Asia No. 12(1). Medicinal Plants 1. Prosea Foundation , Bogor, Indonesia 386-387.
- 12.Padua L S, Bunyapraphatsara N, Lemmens R H. (1999) Medicinal and poisonous plants.1: Prosea Foundation. , Bogor, Indonesia, Plant Resources of South-East Asia 12.
- 13.Kabesh K, Senthilkumar P, Ragunathan R, Kumar R R. (2015) Phytochemical analysis ofCatharanthusroseusplant extract and its antimicrobial activity. , Int J Pure Appl Biosci 3(2), 162-72.
- 14.Tripathi R, Jain S, Herenz N D, Sharma S, Jain P. (2016) Phytochemical analysis and Antibacterial screening ofCalendula officinalis(L) andCatharanthusroseus(L.) against pathogenic bacteria. , Indian Drugs 53(6), 24-31.
- 15.Moghadam S M, Nateghi L. (2015) Evaluation of glucose in fermentation ofCatharanthusroseusL.G. Don. extract by lactic acid bacteria. , Bull. Env Pharmacol Life Sci 4, 81-87.
- 16.Ross I A. (2003) . In:Ross,I.A.(ed.)Medicinal Plants of the World.Humana Press:Totowa, NJ 175-196.
- 17.Zhou M L, Shao J R, Tang Y X. (2009) Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plantCatharanthusroseus(L.) G. Don (Madagascar periwinkle). , Biotechnol Appl Biochem 52, 313-323.
- 18.Ferreres F, Pereira D M, Valentao P, Andrade P B, Seabra R M et al. (2008) New phenolic compounds and antioxidant potential ofCatharanthusroseus. , J. Agric. Food Chem 56, 9967-9974.
- 19.Taylor W I, Fransworth N R. (1975) TheCatharanthusalkaloids: Botany, chemistry, pharmacology and clinical use.Marcel Dekker Inc. , New York
- 20.Chattopadhyay R R. (1999) A comparative evaluation of some blood sugar lowering agents of plant origin. , J Ethnopharmacol 67, 367-72.
- 21.El-Sayed A, Cordell G A. (1981) XXXIV. Catharanthamine, a new antitumor bisindole alkaloid fromCatharanthusroseus.J. , Nat Prod 44, 289-93.
- 22.El-Sayed A, Handy G A, Cordell G A. (1983) XXXVIII. Confirming structural evidence and antineoplastic activity of the bisindole alkaloids leurosine-N’b-oxide (pleurosine), roseadine and vindolicine fromCatharanthusroseus. , J Nat Prod 46, 517-27.
- 23.Johnson I S, Armstrong J G, Gorman M, Burnett J P. (1963) The Vinca alkaloids: A new class of oncolytic agents. , Cancer Res Jr 23, 1390-427.
- 24.Ueda J Y, Tezuka Y, Banskota A H, Q Le Tran, Tran Q K. (2002) Antiproliferative activity of vietnamese medicinal plants. , Biol Pharma Bull 25, 753-60.
- 25.Singh S N, Vats P, Suri S, Shyam R, Kumria M M. (2001) Effect of an antidiabetic extract ofCatharanthusroseuson enzymatic activities in streptozotocin induced diabetic rats. , J Ethnopharmacol 76, 269-77.
- 26.Heijden R V, Jacobs D I, Snoeijer W, Hallard D, Verpoorte R. (2004) TheCatharanthusalkaloids: Pharmacognosy and biotechnology. , Curr Med Chem 11, 607-28.
- 27.Verpoorte R, Heijden R Van der, Van Gulik WM, Ten Hoopen HJG. (1991) Plant biotechnology for the production of alkaloids present status and prospects. In: Brossi, A.(ed). The Alkaloids Academic Press. , San Diego 40, 1-187.
- 28.Zhao J, Verpoorte R. (2007) Manipulating indole alkaloid production byCatharanthusroseuscell cultures in bioreactors: From biochemical processing to metabolic engineering. , Phytochemistry Reviews 6, 435-457.
- 29.Misawa M. Bio International Inc (1994) Plant Tissue Culture: An Alternative for Production of useful metabolites (FAO Agricultural services Bulletin). , Toronto, Canada 18, 19.
- 30.Misawa M, Hayashi M, Takayama S. (1985) Accumulation of Antineoplastic Agents by Plant Tissue Cultures. In:. Primary and Secondary Metabolism of Plant Cell Cultures Neumann K.H. (ed.) , Berlin Heidelberg 235-246.
- 31.Smith M. (2002) An in vitro approach to investigate medicinal chemical synthesis by three herbal plants. Plant Cell Tissue Organ Culture,70,105-111.
- 32.Premjet D, Itoh K, Tachibana S. (2002) Stimulation of the production of Podophyllotoxin by biogenetic precursors and elicitor inJuniperuschinensisstem-derived callus cultures. , Pakistan Journal of Biological Science 5, 131-316.
- 33.Kalidass C, Ramasamy Mohan V, Daniel A. (2010) Effect of auxin and cytokinin on vincristine production by callus cultures ofCatharanthusroseusL. , (apocynaceae). Tropical and Subtropical Agroecosystems 12, 283-288.
- 34.Kumar A, Patil D, Rajamohanan P R, Ahmad A. (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungusFusariumoxysporumisolated fromCatharanthusroseus. , PloS one 8(9), 71805.
- 35.Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco cultures. , Physiol Plant 15, 473-497.
- 36.Gamborg O L, Miller R, Ojima K. (1968) Nutrient requirements of suspension cultures of soybean root cells. Experimental cell research. 50(1), 151-158.
- 37.Sreevidya N, Mehrotra S. (2003) Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. , Journal of AOAC international 86(6), 1124-1127.
- 38.Catapan E, Otuki M F, Viana A M. (2001) . In vitro culture ofPhyllanthusstipulantus(Euphorbiaceae). Revista Brasileria Botanica 24, 25-34.
- 39.Catapan E, Marcio L, Silva B, Moreno F N, Viana A M. (2002) Micropropagation, callus and root culture ofPhyllanthusurinaria(Euphorbiaceae). , Plant Cell Tissue and Organ Culture 70, 301-309.
- 40.Liung O P, Lai C H. (2006) In vitro plant regeneration, flowering and fruiting ofPhyllanthusurinaria(Euphorbiaceae). , International Journal of Botany 2, 409-414.
- 41.Kalidass C, Mohan V R. (2009) In vitro Rapid clonal propagation ofPhyllanthusurinariaLinn. , (Euphorbiaceae)- Medicinal Plant Researcher 1(4), 56-61.
- 42.Kadkade P G. (1981) Formation of podophyllotoxins byPodophyllumpeltatumtissue cultures. , Naturwissenschaften 68(9), 481-2.
- 43.Kadkade P G. (1982) Growth and podophyllotoxin production in callus tissues ofPodophyllumpeltatum. , Plant Science Letters 25(1), 107-115.
- 44.Drewes F E, J Van Staden. (1995) Initiation of and solasodine production in hairy root cultures ofSolanummauritianumScop. Plant Growth Regulation. 17(1), 27-31.
- 45.Rosli N, Maziah M, Chan K L, Sreeramanan S. (2009) Factors affecting the accumulation of 9-methoxycanthin-6-one in callus cultures ofEurycomalongifolia. , Journal of Forestry Research 20(1), 54-8.
- 46.Kittipongpatana N, Hock R S, Porter J R. (1998) Production of solasodine by hairy root, callus, and cell suspension cultures ofSolanumaviculareForst. , Plant Cell, Tissue and Organ Culture 52(3), 133-43.
- 47.Verma A K, Singh R R, Singh S. (2012) Improved alkaloid content in callus culture ofCatharanthusroseus. , Bot. Serbica 36(2), 123-30.
- 48.Aly U I, El-Shabrawi H M, Hanafy M. (2010) Impact of culture conditions on alkaloid production from undifferentiated cell suspension cultures of Egyptian henbane. , Aust J Basic Appl Sci 4, 4717-4725.
- 49.Panda A K, Bisaria V S, Mishra S. (1992) Alkaloid production by plant cell cultures ofHolarrhenaantidysenterica: II. Effect of precursor feeding and cultivation in stirred tank bioreactor. , Biotechnology and Bioengineering 39(10), 1052-1057.
- 50.Ahmad S, Garg M, Tamboli E T, Abdin M Z, Ansari S H. (2013) In vitro production of alkaloids: Factors, approaches, challenges and prospects. Pharmacognosy Reviews.7(13). 27.
- 51.Zhong J J. (2001) Biochemical Engineering of the Production of Plant-Specific Secondary Metabolites by Cell Suspension Cultures. In:. Zhong J.J. et al. (eds). Plant Cells. Advances in Biochemical Engineering/Biotechnology, vol 72.Springer , Berlin, Heidelberg,pp 1-26.
- 52.Vanisree M, Lee C Y, Lo S F, Nalawade S M, Lin C Y. (2004) Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. , Bot Bull Acad Sin 45(1), 1-22.
- 53.Constabel F, Gaudet-LaPrairie P, WGW Kurz, Kutney J P. (1982) Alkaloid production inCatharanthusroseuscell cultures. , Plant Cell Reports 1(4), 139-142.
- 54.Decendit A, Liu D, Ouelhazi L, Doireau P, Mérillon J M.Rideau M.(1992) Cytokinin-enhanced accumulation of indole alkaloids inCatharanthusroseuscell cultures—the factors affecting the cytokinin response. , Plant Cell Reports 11(8), 400-403.
- 55.Tom R, Jardin B, Chavarie C, Archambault J. (1991) Effect of culture process on alkaloid production byCatharanthusroseuscells: I. Suspension cultures.Journal of biotechnology,21(1-2):. 1-19.
- 56.Tom R, Jardin B, Chavarie C, Rho D, Archambault J. (1991) Effect of culture process on alkaloid production byCatharanthusroseuscells: II. Immobilized cultures.Journal of biotechnology.21(1-2):. 21-42.
- 57.Petersen K K, Hansen J, Krogstrup P. (1999) Significance of different carbon sources and sterilization methods on callus induction and plant regeneration ofMiscanthus xogiformisHonda Giganteus'. Plant cell, tissue and organ culture. 58(3), 189-197.
- 58.Amir M, Aqil M, Ismail M V, Akhtar M, Khan A H. (2017) Effect of carbon source and inubation temperature on total content of secondary metabolites of callus culture ofSolanum nigrum.World. , Journal of Pharmaceutical Research 6(8), 905-922.
- 59.Bondarev N, Reshetnyak O, Nosov A. (2003) Effects of nutrient medium composition on development ofSteviarebaudianashoots cultivated in the roller bioreactor and their production of steviol glycosides. , Plant Science 165(4), 845-850.
- 60.Mishra M R, R K Srivastava, Akhtar N. (2018) Enhancing alkaloid production from cell culture system of Catharanthusroseus with different carbon sources. , European Journal of Biotechnology and Bioscience 6(5), 12-20.
- 61.Lee C W, Shuler M L. (2000) The effect of inoculum density and conditioned medium on the production of ajmalicine and catharanthine from immobilizedCatharanthusroseuscells. Biotechnology and bioengineering. 67(1), 61-71.
- 62.Yokoyama M, Inomata S, Seto S, Yanagi M. (1990) Effects of sugars on the glucosylation of exogenous hydroquinone byCatharanthusroseuscells in suspension culture. Plant and cell physiology. 31(4), 551-555.
- 63.Sakuta M, Takagi T.Komamine A.(1987) Effects of sucrose on betacyanin accumulation and growth in suspension cultures ofPhytolaccaamericana. , Physiologia Plantarum 71(4), 455-458.
Cited by (42)
This article has been cited by 42 scholarly works according to:
Citing Articles:
Saptak Sarkar, Swati Sharma - South African Journal of Botany (2025) Semantic Scholar
South African Journal of Botany (2025) OpenAlex
South African Journal of Botany (2025) Crossref
Elsevier eBooks (2024) OpenAlex
J. G. Patil, T. Nikam, R. A. Shinde, Mahendra Laxman Ahire - Discover Plants (2024) Semantic Scholar
Discover Plants. (2024) OpenAlex
Discover Plants (2024) Crossref
Siddharth Goswami, Amena Ali, M. E. Prasad, Pallavi Singh - Pharmacological Research - Modern Chinese Medicine (2024) Semantic Scholar
Pharmacological Research - Modern Chinese Medicine (2024) OpenAlex
Pharmacological Research - Modern Chinese Medicine (2024) Crossref
Food bioactive ingredients (2023) OpenAlex
Mahboobeh Yazdi, A. Bagheri, N. Moshtaghi, Fatemeh Keykha Akhar, Azade Khadem - Journal of Medicinal Plants (2023) Semantic Scholar
Armaghan. (2023) OpenAlex
Journal of Medicinal Plants (2023) Crossref
Egyptian Pharmaceutical Journal (2023) OpenAlex
Egyptian Pharmaceutical Journal (2023) Crossref
M. Martínez, Lorena Jorquera, P. Poirrier, Katy Díaz, R. Chamy - Plants (2023) Semantic Scholar
Plants (2023) OpenAlex
Plants (2023) Crossref
Ahmed ElTantawy - Scientific Journal of Agricultural Sciences (2023) Semantic Scholar
Scientific Journal of Agricultural Sciences (2023) OpenAlex
Horticulture, Environment, and Biotechnology (2022) Crossref
Sima Sazegari, A. Niazi, Farajollah Shahriari-Ahmadi, A. Afsharifar - Horticulture Environment and Biotechnology (2022) Semantic Scholar
Horticulture Environment and Biotechnology (2022) OpenAlex
Horticulturae (2022) OpenAlex
Horticulturae (2022) Crossref
Beni-Suef University Journal of Basic and Applied Sciences (2022) OpenAlex
Beni-Suef University Journal of Basic and Applied Sciences (2022) Crossref
M. T. Khandy, Anastasia K. Sofronova, T. Gorpenchenko, N. Chirikova - Plants (2022) Semantic Scholar
Santoshi Acharjee, Raghawendra Kumar, Nitish Kumar - Industrial crops and products (Print) (2022) Semantic Scholar
Industrial Crops and Products (2022) Crossref
M. Miransari, Shirin Adham, Mahdiar Miransari, Arshia Miransari - Applied Microbiology and Biotechnology (2022) Semantic Scholar
Applied Microbiology and Biotechnology (2022) OpenAlex
Applied Microbiology and Biotechnology (2022) Crossref
Tran My Linh, N. Mai, P. T. Hoe, N. Ngoc, Phan Thi Hong Thao et al. - Plants (2021) Semantic Scholar
Plants (2021) OpenAlex
Plants (2021) Crossref
Munirah Adibah Kamarul Zaman, A. M. Azzeme, I. K. Ramle, Nurfazlinyana Normanshah, N. A. Shaharuddin et al. - In Vitro Cellular & Developmental Biology - Plant (2021) Semantic Scholar
In Vitro Cellular & Developmental Biology - Plant (2021) OpenAlex
In Vitro Cellular & Developmental Biology - Plant (2021) Crossref
R. Jeyapackiaseeli, T. D. Kumar - Soft Computing for Intelligent Systems (2021) Semantic Scholar
Algorithms for intelligent systems (2021) OpenAlex
Industrial Crops and Products (2021) OpenAlex
International Journal of Secondary Metabolite (2019) OpenAlex
International Journal of Secondary Metabolite (2019) Crossref
M. Rady - Plant Biotechnology and Medicinal Plants (2019) Semantic Scholar
Agung B S Satyarsa - Journal Of Medicine & Health (2019) Semantic Scholar
Journal Of Medicine & Health (2019) OpenAlex
