First Evidences of Epithelial-Mesenchymal Transition and Cancer Stem-Cell Phenotype Acquisition in Dermo-Epidermal Junction of BPV-Infected Neoplasms
Abstract
Introduction
Bovine papillomavirus (BPV) is the etiological agent of bovine papillomatosis, infectious and neoplastic disease, characterized by the presence of multiple papillomas that can regress spontaneously or to persist and progress to malignancies when in association with environmental cofactors. Although recognized that the BPV can induce DNA damages, the viral role following cancer initiation remains unresolved. Based on this, we stablished cell lines derived from cutaneous papilloma, fibropapilloma and esophageal carcinoma to study the BPV action on epithelial-mesenchymal transition (EMT). Our results showed strong evidences that the virus action can contribute to EMT and, therefore, metastasis.
Aim
In this study, we analyzed the expression levels of the EMT markers (cytokeratin 10, STAT3 Y705, Oct-3/4 and vimentin) in paraffin-embed samples, using the same tissues that originated the cell lines previous studied, aiming to validate the results observed using cell lines.
Material and Methods
Expression levels of these markers was analyzed by immunohistochemistry and the collagen composision by Picrosirius red staining.
Results
We verified an overexpression of these markers in fibroblastoid cells present into the epidermis and ketarinocyte-like cells into the dermis present in dermo-epidermal junction. These data reinforce our previous results using cell cultures, validating both systems (cell culture and paraffin-embed tissues) as useful models to study the natural history of BPV-infected lesions.
Conclusion
Altogether, the results from these systems indicate that the BPV promote the cancer progression and metastasis through the transdifferentiation of an epithelial to mesenchymal cells (EMT).
Author Contributions
Academic Editor: Syed Shams ul Hassan, Ocean college Zhejiang University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Rodrigo Pinheiro Araldi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Bovine papillomavirus (BPV) is a worldwide oncogenic virus, present in at least 60% of Brazilian cattle herd 1, 2. The BPV is the etiological agent of bovine papillomatosis (BP), infectious and neoplastic disease, characterized by the presence of multiple papillomas (warts) that can regress spontaneously or to persist and progress to urinary bladder or upper alimentary tract carcinoma when in association with environmental cofactors, such as the quercetin and ptaquilosides found in the break fern Pteridium ssp.2, 3, 4, 5.
Although recognized that the BPV promotes cytogenetic aberrations 1, 6, which are related to the viral clastogenic potential 7, 8, 9, the viral action following cancer initiation remains few explored 10. The lack of studies about the BPV on cancer progression and metastasis can be attributed to the lack of attention given to cell cultures derived from BPV-infected tissues, which are mandatory to interrogate the viral action following cancer initiation.
In this sense, since 2008, when we established with success different cell lines derived from BPV-infected benign and malignant neoplasms 11, we have been explored the potential of these cell lines as model to study the BPV-related oncogenic process. Using these cell lines, we already showed that they exhibit cytogenetic alterations 3, 12 similar to those verified in short-term lymphocyte cultures derived from BPV-infected animals 1, 6, 7. Similar data were also described in primary cell cultures derived from BPV-infected papilloma and equine sarcoid 13. These results suggest that cell lines derived from BPV-infected neoplasms are useful model to study the viral action on cancer initiation.
Currently we also described the BPV L1 capsid protein expression and the presence of virus-like particles in cytoplasmic vesicles of cells in sixth passage derived from cutaneous papilloma, fibropapilloma and esophageal carcinoma 14, suggesting that these cell lines is also an useful model to study the viral biology. In addition, we verified that BPV can induce metabolic alterations in cell lines derived from fibropapilloma and esophageal carcinoma as a possible consequence of anti-oxidant action of BPV E6 oncoprotein 12. These deregulations in energetic metabolism lead to reactive oxygen species (ROS) production, inducing the DNA oxidation. This action can result in DNA breaks (clastogenesis) and, to activate the nuclear transcription factor STAT3 through the phosphorylation of tyrosine residue 705 (Y705) 15. When activate, the STAT3 Y705 promotes cell proliferation 16 by the activation of mitogen-activated protein kinase (MAPK) 17 and platelet-derived growth factor beta (PDGFβ) upregulation 18. The STAT3 activation also induces the mesenchymal proteins expression, including vimentin and, regulates the cell differentiation 19, 20. Thus, these data can justify the overexpression of STAT3 Y705, Oct-3/4 and vimentin verified in cell lines derived from BPV-infected neoplasms 21. Altogether, these alterations suggest that BPV infection persistence can lead to epithelial-mesenchymal transition (EMT). The EMT is a complex pathological process verified during metastasis, characterized by reversible metabolic, genetic and morphological reprograming 10, 20, 22, 23, which can lead to cancer stem-cell (CSC) formation 23, 24, 25, 26.
Based on these data, this study aimed to evaluate the expression of cytokeratin 10 (CK10), STAT3 Y705, Oct-3/4 and vimentin in paraffin-embed tissue samples from BPV-infected cutaneous papilloma (papilloma 01), fibropapilloma (papilloma 02 and 03) and esophageal carcinoma and BPV-free normal skin (negative control) that originated the cell lines used in previous studies to validate the results previous verified in cell culture.
Material and Methods
Ethics Statement
This study was approved by the Ethic Committee on Animal Use of Butantan Institute (process number 1319/14).
Tissue Obtaining
Three samples of skin warts and one of esophageal carcinoma were collected from four different adult bovines (Simmental breed). One samples of healthy skin was collected from a calf with eight months (Simmental breed) without visible papillomas or clinical sign of BPV infection, which was used as negative control. These samples were collected by a veterinarian using 2% lidocaine. Each sample was divided in two fragments, which were destined to histopathological and immunohistochemical analysis. The BPV DNA molecular identification was performed by PCR and showed the absence of viral sequences in normal skin and the co-infection by BPV-1, 2 and 4 in samples from skin warts and esophageal carcinoma, as previous described by Araldi et al. 12 using cell lines derived from these tissues.
Histopathological Analysis
The fragments destined to histopathological and immunohistochemical analysis were fixed at 10% formalin, dehydrated in increasing concentrations of ethanol, embedded in paraffin and cut in 4 µm sections. For the histopathological analysis, the material was stained with hematoxylin-eosin (HE). The slides were analyzed in binocular optical microscope Axiophot (Carl Zeiss, Germany) with the objectives of 5X, 10X, 20X and 40X and the images were captured using the AxioVision software version 4.7.2. (Carl Zeiss, Germany). The paraffin embed tissues are part of the biological collection of Butantan Institute.
Table 1. Results of collagen composition of extracellular matrix| % slide area | % cut area | |||
| Sample | Type I | Type III | Type I | Type III |
| BPV-free normal skin | 14,770 | 75,855 | 16,30 | 83,70 |
| Papilloma 01 | 42,806 | 24,040 | 64,04 | 35,96 |
| Papilloma 02 (FP) | 53,365 | 22,226 | 70,59 | 29,41 |
| Papilloma 03 (FP) | 22,075 | 47,870 | 82,18 | 17,82 |
| Esophageal carcinoma | 25,839 | 52,370 | 83,15 | 16,85 |
Immunodetection of BPV E5 Oncoprotein, CK10, STAT3, Oct-3/4 and Vimentin
The expression of BPV E5 oncoprotein, CK10, STAT3, Oct-3/4 and vimentin was evaluated through immunohistochemical analysis (IHQ). For this, the paraffin embed tissues were subjected to cuts of 4 µm using microtome and transferred to silanised slides. Sections were deparaffinized using the following method: 30 minutes in xylene 100% for twice, 30 minutes in 1:1 (v/v) etnahol-xylene, 10 minutes in ethanol absolute for twice, two minutes in ethanol 95º and two minutes in ethanol 70º. Next, the sections were treated for 10 minutes with a 10% ammonium hydroxide solution in ethanol 95º and washed three times with distillated water for five minutes. The antigen retrieval was performed in microwave using the 0.01 M citrate buffer for five minutes at 750 W and five minutes at 450 W. After these steps, the slides were immediately transferred to container containing ice for the solution cooling (about 40 minutes). The immunodetection of CK10, Oct-3/4 and vimentin was performed using the EnVisionTM + HRP System (Dako, USA), while the immunodetection of BPV E5 oncoprotein and STAT3 was performed using in house method. For both methods, the endogenous peroxidase was blocked with the peroxidase block, included in the EnVision kit for 10 minutes. Sections were washed three times with TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) for five minutes, treated with 0.01% Triton X-100 for 20 minutes and next, washed for five minutes with TBS. The tissue was blocked with 5% BSA for 40 minutes and washed for five minutes with TBS. Next, the material was incubated overnight with the primary antibodies (Table 2) at 4ºC in humid chamber. Slides were washed twice with TBS-T (TBS, 0.2% Tween-20) and, the material was incubated with the secondary antibody (Table 2) for one hour at 4ºC in humid chamber. Sections were washed three time with TBS-T for five minutes and treated with the chromogenic substrate 3-amino-9-ethylcarbazole 1 (AEC) for 15 minutes (for CK10, Oct-3/4 and vimentin antibodies) and 3, 3’-diaminobenzidine (DAB) (Dako, USA) for five minutes (for BPV E5 oncoprotein and STAT3 antibodies). The slides were stained with Mayer-hematoxylin for three minutes and washed three times in distillated water. The slides subjected to the immunodetection using the EnVision kit were mounted with the Faramount mounting medium (Dako, USA). The slides subjected to in house immunodetection were treated with ethanol 70º, ethanol 95º, ethanol absolute for three minutes, 1:1 (v/v) ethanol-xylene for 10 minutes, xylene 100% for 10 minutes and mounted using Entellan (Merck, Germany). The slides were analyzed using the Axiophot (Carl Zeiss, Germany) binocular microscope employing objectives of the 10X, 20X, and 40X. Images were captured using the AxioVision software version 4.7.2. (Carl Zeiss, Germany). A fragment of skin tissue from a BPV-free calf (experimental control) and a fragment of papilloma without the addition of primary antibody (reaction control) were used.
Table 2. Antibodies employed for immunohistochemistry analyses| Antibody | Specificity | Produced | Reference | Dillution |
|---|---|---|---|---|
| Anti-E5*, 1 | pAb | sheep | - | 1:500 |
| Anti-CK102 | mAb | mouse | Dako (M7002) | 1:200 |
| Anti-STAT3 Y7053 | pAb | rabbit | Immuny (IM0448) | 1:100 |
| Anti-Oct-3/42 | mAb | mouse | S. Cruz | 1:200 |
| Anti-vimentin2 | mAb | mouse | Dako (M0725) | 1:200 |
Analysis of Collagen Composition
Considering that the EMT is also characterized by alterations in extracellular matrix, this study also evaluated quantitatively the collagen composition using the Picrosirius red coloring. This method allows to identify collagen fibers of type I, which present red-orange color, whereas collagen fibers of type III, yellow-green color 27. The cuts were subjected to dewaxing, hydrated and colored for 40 minutes with Picrosirius red. Next, the slides were immersed in aqueous solution of 0.5% of sodium hydroxide. The cuts were colored with hematoxylin and washed in distillated water. Coverslips were mounted on slides using the Tissue Teck medium (Sakura Finetek, Netherlands). Slides were analyzed in binocular microscope Olympys Bx51 in bright field using the polarizer Olympus 45 MM for transmitted light different magnification (50-400X). Collagen fiber quantification was obtained based on the area percentage using the ImageJ software, according to Hadi et al. 28.
Results
Histopathological Analysis
Histopathological analysis showed the preservation of tissue architecture of normal skin, the absence of atypia and dysplasia and the presence of sebaceous gland and hair follicle into the dermis (Figure 1). By the contrast, the three samples of skin warts showed a notorious hyperkeratosis, the presence of mitotic keratinocytes and a consecutive hyperplasia of suprabasal layer (acanthosis) (Figure 1). It was also verified the presence of koilocytes into the suprabasal layer, cells characterized by the presence of a prominent halo and acentric nucleus (Figure 1). The dermis of these warts showed a diffuse inflammatory infiltrate close to blood vessels (Figure 1). However, the samples of papilloma 02 and 03 showed a fibro-elastic dermis, exhibiting an expressive fibroblastic proliferation, with cell dispose in fascicular bundles (Figure 1). Due to these characteristics, the papillomas 02 and 03 were classified as fibropapilloma, while the papilloma 01, as cutaneous papilloma.
Figure 1.Histopathological analysis showing the tissue architecture preservation in BPV-free normal skin. The cutaneous papilloma, fibropapilloma (FP) and esophageal carcinoma showed acanthosis and koilocytosis, characteristics even verified in BPV-infected lesions. The samples of papilloma 02 and 03 showed a fibroelastic and reactive dermis, due to these characteristics, these samples were classified as fibropailloma (FB). Both cutaneous papilloma and fibropapilloma showed an extensive inflammatory infiltrate into the dermis. Esophageal carcinoma exhibited the sites of atypia, where was verified the loss of cell polarity, the presence of cancer cell islands into the dermis, comprised by fibroblastoid keratinocytes, being compatible with epithelial-mesenchymal transition (EMT).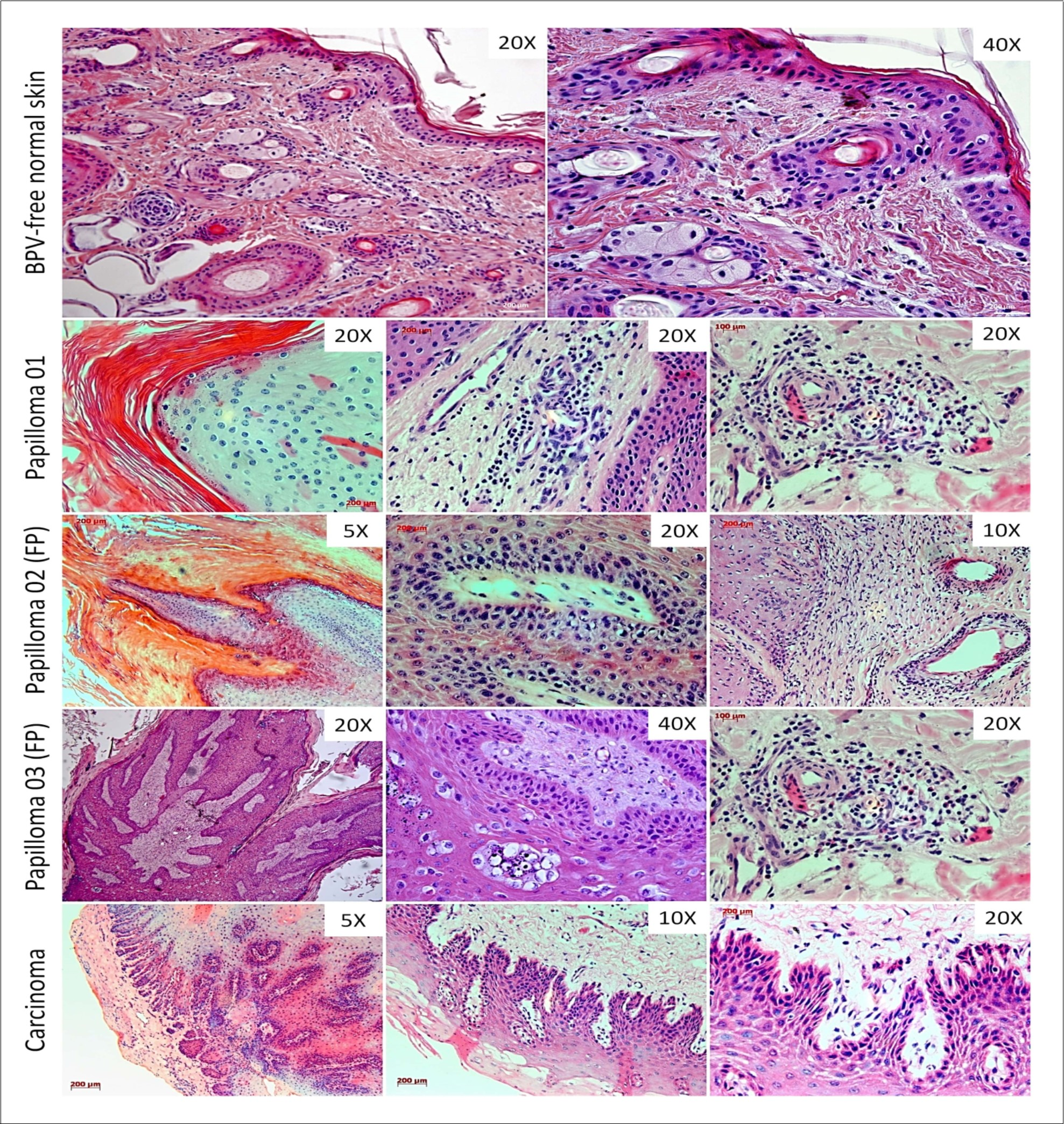
The esophageal carcinoma sample showed and evident tissue disorganization, with the presence of epithelioid cell islands into dermis and fibroblastoid cells in the invasion front, suggesting the loss of cell polarity (Figure 1).
Immunodetection of BPV E5 Oncoprotein
Through the immunohistochemical analysis, it was detected the plasmatic membrane and cytoplasmic expression of BPV E5 oncoprotein in suprabasal epithelium layer of cutaneous papilloma, fibropapilloma and esophageal carcinoma (Figure 2). This result reinforces the BPV infection in these tissues. By the opposite, it was not verified the expression of this oncoprotein in normal skin, reinforces the PCR results, indicating the absence of viral infection (Figure 2). It was not verified any labelled in negative control (cutaneous papilloma only incubated with secondary antibody), indicating the antibody specificity (Figure 2).
Figure 2.Immunodetection of BPV-1 E5 oncoprotein showing the absence of labeling in BPV-free normal skin (A), reinforcing the absence of virus infection in this tissue and, in negative control (B, cutaneous papilloma incubated exclusively with secondary antibody). The samples from cutaneous papilloma (C, papilloma 01), fibropapilloma (D and E – papilloma 02 and 03, respectively) and esophageal carcinoma (F) showed an evident expression of BPV-1 E5 oncoprotein, which was detected in both cytoplasm and plasmatic membrane, being in accordance with the protein biology, since the E5 is a hydrophobic transmembrane protein. Images obtained using objectives of 5X (B), 10X (A) and 20X (C-F).
Immunodetection of Cytokeratin 10 (CK10)
Results showed the expression of CK10 in epidermis of BPV-free normal skin and an overexpression of this protein in cutaneous papilloma (papilloma 01) (Figure 3). However, it was verified a reduction of CK10 expression levels in fibropapilloma (papilloma 02 and 03) and esophageal carcinoma samples (Figure 3). These data suggest an acquisition of stem-cell phenotype, since this protein is related to keratinocyte differentiation. It was not verified any labelled in negative control (cutaneous papilloma incubated exclusively with the secondary antibody) (Figure 3).
Figure 3.Immunodetection of cytokeratin 10 (CK10) showing the protein expression restricted to the epithelium of BPV-free normal skin (A), reinforcing the tissue architecture preservation, and the absence of labeling in negative control (B, cutaneous papilloma incubated exclusively with secondary antibody).Results show an expressive immunodetection of CK10 in suprabasal layer of cutaneous papilloma (C). However, was verified a downregulation of CK10 expression levels in fibropapilloma (D and E – papilloma 02 and 03, respectively) and esophageal carcinoma (F). Images obtained using objectives of 5X (B, C, E and F), 10X (D) and 20X (A).
Immunodetection of STAT3 Y705
Results showed an expressive nuclear labelling of STAT3 Y705 in both epidermis and dermis of cutaneous papilloma (papilloma 01) and fibropapilloma (papilloma 02 and 03) (Figure 4). This labelling was verified especially in basal keratinocytes and epithelioid cells into the dermis of these samples present in dermis-epidermis junction (Figure 4). However, the esophageal carcinoma showed a notorious expression of STAT3 Y705 in basal keratinocytes, but not into dermis (Figure 4). This data suggest that the STAT3 action occurs in pre-neoplastic lesions. By the contrast, it was not verified the STAT3 Y705 in BPV-free normal skin (Figure 4). In addition, it was not verified any labelling in the negative control (cutaneous papilloma incubated exclusively with secondary antibody), indicating the absence of unspecific labelling (Figure 4).
Figure 4.Immunodetection of STAT3 Y705 (active) showing the absence of expression in negative control (A, cutaneous papilloma incubated exclusively with secondary antibody) and BPV-free normal skin (B). Results show the aberrant nuclear immunodetection of STAT3 Y705 cutaneous papilloma (C and D, papilloma 01), fibropapilloma (E and F - papilloma 02 and 03, respectively) and esophageal carcinoma (G and H). The STAT3 Y705 expression was verified in keratinocyte-like cells present into the dermis. Images obtained using objectives of 10X (A, B, C and G) and 20X (D, E, F and H).
Immunodetection of Oct-3/4
Results showed the immunodetection of nuclear transcription factor Oct-3/4 in BPV-free normal skin, however, this labelling was restrict to hair follicle cells and some basal keratinocytes (Figure 5). Cutaneous papilloma (papilloma 01) and fibropapilloma (papilloma 02 and 03) samples showed an expressive nuclear labelling of Oct-3/4 in both keratinocytes and epithelioid cells present in dermis and dermis-epidermis junction (Figure 5). Sample of esophageal carcinoma did not show the Oct-3/4 immunodetection (Figure 5). This result can be attributed to the high number of mitotic cells verified in this tissue (Figure 5). Negative control (cutaneous papilloma incubated exclusively with the secondary antibody) showed no labeling, indicating the absence of unspecific labelling (Figure 5).
Figure 5.Immunodetection of Oct-3/4 showing the absence of expression of this nuclear transcription factor in negative control (A, cutaneous papilloma incubated exclusively with secondary antibody) and the Oct-3/4 expression restricted to cells of hair follicle (pointed by the arrow) and some basal keratinocytes of BPV-free normal skin, as expected (B and C). However, the results show an aberrant nuclear immunodetection of Oct-3/4 in both epidermis and dermis of cutaneous papilloma, including in depolarized basal keratinocytes (arrow) (D, papilloma 01) and fibropapilloma (E – papilloma 02 and F-G – papilloma 03). It was not verified the Oct-3/4 expression in esophageal carcinoma (H and I). This result can be attributed to the high mitogenic activity observed in this tissue, which was verified by the presence of mitotic cells (arrow) (I). Images obtained using objectives of 5X (C, E and H), 10X (A, D, F and I) and 20X (B and G).
Immunodetection of Vimentin
Results showed the immunodetection of mesenchymal marker vimentin into the dermis of BPV-free normal skin, cutaneous papilloma (papilloma 01), fibropapilloma (papilloma 02 and 03) and esophageal carcinoma (Figure 6). However, it was observed the vimentin expression in keratinocytes of dermo-epidermal junction and suprabasal layer of all BPV-infected tissues (cutaneous papilloma, fibropapilloma and esophageal carcinoma), but not in BPV-free normal skin (Figure 6). These labeling were verified especially in fibroblastoid cells of dermo-epidermal junction, suggesting the acquisition of mesenchymal phenotype verified during the EMT. The negative control (cutaneous papilloma incubated exclusively with secondary antibody) showed the absence of unspecific labeling (Figure 6).
Figure 6.Immunodetection of vimentin showing the absence of labeling in negative control (cutaneous papilloma incubated exclusively with secondary antibody) and the protein expression into the dermis of BPV-free normal skin, but not in the epithelium. Results show the vimentin immunodetection in both dermis, as expected, and epidermis of all BPV-infected lesions, including esophageal carcinoma. It is verified the vimentin expression in koilocytes and fibroblastoid keratinocytes present in dermoepidermal junction and suprabasal layer.
Analysis of Collagen Composition
Analysis of collagen composition showed a reduction of collagen fibers type I in cutaneous papilloma (papilloma 01), fibropapilloma (papilloma 02 and 03) and esophageal carcinoma in relation to normal skin (Figure 7, Table 1). These data suggest that BPV infection can lead to extracellular matrix (ECM) remodeling, contributing to cell migration and, therefore, which EMT.
Figure 7.Analysis of collagen composition of extracellular matrix. Results show the prevalence of fibers type III in BPV-free normal skin (A) and the predominance of fibers type I in cutaneous papilloma (B, papilloma 01), fibropapilloma (C and D, papilloma 02 and 03, respectively) and esophageal carcinoma (E). Quantitative analysis using the software ImageJ, showing the increase of collagen type I in BPV-infected lesions. Images obtained using the objective of 5X.
Discussion
In last years, studies involving the EMT and CSC formation have been awakened attention. This because the EMT and CSC are intrinsically associated, since the biochemical and genetic reprograming that lead to the mesenchymal phenotype acquisition can also promote the cell dedifferentiation, resulting in the stem-cell acquisition 24, 25, 29. However, while the cell reprograming that lead to EMT is dependent on reversible epigenetic changes, the genetic alterations verified in this process can be irreversible, contributing with the CSCs development 30.
The CSCs represent one of the major challenges to be overcome in oncology field, once these cells are refractory to chemotherapeutics 31 and are related to the metastasis formation 32. Considering the Darwinian dynamic of cancer, the acquisition of both mesenchymal and stem-cell phenotype confers competitive advantages for CSCs, which exhibit a most efficient repair system, making these cells less sensitive to antineoplastic drugs and/or radiotherapy 32, 33. These data are alarming, since the CSCs can comprise until 25% of the cancer cell content 34. Moreover, due to the capability to divide asymmetrically, these cells can self-renewal, maintain these cell subpopulation into tumor microenvironment 35.
The genomic instability is recognized as drives the carcinogenesis by induce the cancer initiation 36, 37. However, current studies have been also shown that genomic instability is related to CSCs formation 38, 39. In this sense, the BPV is an oncogenic virus known as induce DNA damages in host chromatin, leading to genomic instability 1, 7, 40. Despite this, the BPV action following cancer initiation remains unresolved 10. The lack of studies about the viral role in metastasis can be attributed to the replication viral paradigm, which is dependent on epithelium differentiation 41. For this reason, little attention has been given to cell lines derived from BPV-infected neoplasms. Moreover, studies involving paraffin embed tissues focus on the immunodetection of viral proteins or oncoproteins and their interaction with host proteins 9, 42, 43.
In this context, in 2008 we established cell lines derived from BPV-infected cutaneous papilloma, fibropapilloma and esophageal carcinoma 11, bringing new opportunities to interrogate the BPV action in cancer progression and metastasis. Using these cell lines, we already verified that the viral infection persistence can lead promote the glycolytic metabolism activation and increase the ROS production 12. We also verified that BPV induces the activation and overexpression of STAT3 and Oct-3/4, leading to the loss of cell polarity, cell migration and formation of oncospheres 12, 21. Now, we investigate if these alterations are also present in the tissue samples that originated these cell lines.
First, we analyzed the tissue architecture through histopathological analysis. Results of this analysis showed the preservation of epithelial and dermal histology of normal skin (Figure 1). Considering the results of PCR and BPV E5 immunodetection (Figure 2 and Figure 3, respectively), we confirmed the absence of BPV infection in this sample, allowing its use as negative control. However, the samples of papilloma 01-03 showed a notorious hyperplasia of suprabasal layer (acanthosis) (Figure 1). Acanthosis is consequence of proliferative action of E5, E6 and E7 oncoproteins, that combined stimulates the S phase entry, making available the DNA polymerases required for BPV replication. Considering that BPV is able to promote the oxidative stress 10, 12, acanthosis can be also discussed as a consequence of ROS production, which, under determinate concentrations, can lead to cell proliferation as previously described in equine sarcoid infected by BPV-1 and 2 44 and in bovine fibropapilloma 9.
The samples of papilloma 01-03 also showed a hyperkeratosis (Figure 1). Hyperkeratosis confers a physical protection for virions, at the same time that avoid an immune response, since the icosahedral morphology of BPV capsid is immune-reactive. 2. The papilloma 03 also showed a high quantity of kerato-hyaline granules on granular layer (Figure 1). The presence of these granules was first identified in 1869 by Auffhame and, later, described in 1873 by Langerhans 45. These granules are rich of cysteine, amino acid characterized by the presence of sulfhydryl group responsible for the disulfide bond, involved in keratinization process 45, 46.
Considering the natural history of BPV replication cycle, the viral particles are release from the keratinocytes in degeneration present in suprabasal and granular layer. These cells exhibit a prominent halo and an acentric nucleus, being known as koilocytes 47, 48. The presence of these cells is considered for some authors as a pathognomonic marker of papillomavirus infection 47, 49. Thus, the koilocytes verified in papillomas 01-03 samples indicate the BPV infection in these tissues, which was proved by the PCR results and the BPV E5 oncoprotein immunodetection (Figure 2 and Figure 3, respectively). Koilocytosis represents a cytopathic effect of viral infection 50, being observed in papillomas of different species, including humans 47, 48, 51, 52. Although the pathways involved in perinuclear vacuolization are unclear, it is believed that it could contribute to cell fragility, facilitating the virion release to environment 53.
However, the samples of papilloma 02 and 03 showed a fibro-elastic reactive stroma, characterized by the intense fibroblastic proliferation (Figure 1). For this reason, these samples were classified as fibropapilloma. Fibropapilloma is histologically similar to equine sarcoid, fibroblastic benign neoplasm, locally invasive but not metastatic, that until 1963 was known as equine fibropapilloma 54, 55, 56, 57. Fibropapilloma are associated to BPV-1, 2 and 13 (Deltapapillomavirus) and BPV-5 (Epsilonpapillomavirus) infection, being also described in zebra (Equus burchelli), horse, buffalo llama, camel, alpaca, dumb and cats infected by BPV, as well as by ovine infected by OvPV-1 and 2 56, 58, 59, 60, 61, 62, 63.
Esophageal carcinoma showed a tissue disorganization (Figure 1), which can be attributed to cell proliferation. Histopathological results also showed a reactive dermis, whith epithelioid cells islands in dermaepidermla junction (Figure 1). These islands suggest an invasiviness and migratory phenotype confered by the EMT. Furtheremore, it was verified the presence of fibroblastoid cells into these islands (Figure 1), indicating the loss of apical-basal polarity, suggesting the acquisition of EMT phenotype. Similar results were described in the cell lines derived from this tissue 21.
Aiming to confirm the BPV infection, we performed the immunodetection of E5 oncoprotein. The E5 is a small hydrophobic transmembrane protein 64, 65, recognized as the main transforming protein 66. The E5 oncoprotein can bind and promote the PDGFβR dimerization 64, 67, 68. This action lead to activation of different kinases (A-cdk2, MAPK, JNK, PI3K e c-Src), resulting in cell proliferation and cancer progression 64, 69, 70. The E5 oncoprotein also promotes the loss of focal adhesion, leading to invasion 71. Thus, the E5 is also related with EMT and, therefore, with CSC formation.
We verified the E5 oncoprotein expression on plasmatic membrane and cytoplasm of basal and suprabasal keratinocytes of papilloma 01, 02, 03 and esophageal carcinoma samples (Figure 2), but not in BPV-free normal skin (Figure 2). This data reinforces the viral infection, previous verified in cell lines derived from these tissues 12.
Considering that during the EMT occur the repression of epithelial markers, we analyzed the expression levels of cytokeratin 10 (CK10). The CK10 is one the most analyzed cytokeratin in pathology, since it is recognized as a marker of cell differentiation, being expressed from suprabasal to corneal epithelium layer 72, 73, 74. The immunodetection of CK10 has been commonly used in histopathological routine, once alterations in CK10 expression levels are observed in carcinomas 73, 75, 76, 77, 78. These alterations are frequently associated with metastasis in human 75, 77 and bovines 76, making the CK10 a prognostic marker since 1980 75, 77, 79.
Cytokeratin is a class of evolutionary conserved structural proteins 80, encoded by different genes and classified in two types: type I (acid polypeptides with 40-56 kDa, including the CK9 to CK20) and type II (neutral and basic polypeptides with 53-68 kDa, including CK1 to CK8) 75. The CK monomers are fastly degradated 75. For this reason, cytokeratins form heterodimers comprised by type I and II CKs, conferring mechanical stability to the cells 75, 79, 80. Thus, the results showed a notorious reduction in CK10 expression levels in fibropapilloma and esophageal carcinoma in relation to BPV-free normal skin and cutaneous papilloma (Figure 3). This result is very interest, once the fibropapillomas showed an acanthosis similar to cutaneous papilloma (Figure 1), should be expected similar levels of CK10 expression in both cutaneous papilloma and fibropapilloma. Thus, considering that the cancer emerges from a pre-neoplastic lesion, these results suggest that fibropapilloma can be discussed as a pre-malignant lesion. These data are in accordance to the previous results obtained from cell lines derived from the tissues samples used in this study, that showed that fibropapillomas exhibit metabolic alterations 12 and an intermediate migration capability in relation to the BPV-free normal skin and esophageal carcinoma 21. Altogether, these data reinforce our hypothesis that fibropapillomas are pre-malignant lesion.
Results also showed an increase in STAT3 Y705 expression levels in all BPV-infected lesions in relation to BPV-free normal skin (Figure 4). The STAT3 is a member of Signal Transducer and Activator of Transcription (STAT) family of nuclear transcriptional factor 81. These factor regulate different target genes, leading to cell proliferation, differentiation, apoptosis, migration, angiogenesis and anoikis resistance 81. The STAT3 nuclear factor is crucial for the maintenance of tissue integrity, regulating the epithelial-mesenchymal interaction 82, 83. However, the aberrant expression/activation of STAT3 is related to the malignant transformation and metastasis formation 16, 81.
The STAT3 protein remains latent in the cytosol 18, where is activated by the Janus kinase (JAK) 84, which promotes the phosphorilation of (1) 727 serine residue present in the transactivation domain (S727) or (2) 705 tirosine residue presente in C-terminal domain (Y705) 83. Current studies have been shown that the Y705 phosphorilation is the main pathway of STAT3 activation 83, 85. For this reason, we analyzed the levels of expression of STAT3 Y705. We observed an expressive immunodetection of STAT3 Y705 in the dermo-epidermal junction of cutaneous papilloma, fibropapilloma and esophageal carcinoma (Figure 4). Curiously, the cells expression STAT3 Y705 into the epidermis exhibited a fibroblastoid morphology, showing the loss of apical-basal polarity, while cells expressing this factor into the dermis showed a keratinocyte-like morphology (Figure 4). These results are in consonance with our previous data, verified using the cell lines derived from these tissues 12.
The STAT3 activation also lead to the CSC formation 83, justifying the repression of CK10 expression observed in fibropapilloma and esophageal carcinoma (Figure 3). However, the activation of STAT3 is not restricted to cells infected by BPV. Studies have been described that other oncogenic viruses, such as the Epstein-Barr virus (EBV) 86, Rous sarcoma virus (RSV) 87, human T-leukemia virus (HTLV) 88, and the hepatites B (HBV) and C viruses (HCV) 89 can also promote the STAT3 phosphorilation. Altogether, these results suggest that the STAT3 activation is a carcinogenic pathway shared among oncogenic viruses. For this reason, to explore drugs able to modulate this pathway can be considered a plausible alternative to avoid the cancer development or, control the cancer progression and consequent metastasis.
Considering these results, that combined suggest a cell dedifferentiation along the natural history of cancer development, we analyzed the expression levels of Oct-3/4 (POU5F1), which is recognized as marker of CSC 90. The results of this analysis showed an expressive nuclear immunodetection of Oct-3/4 in dermo-epidermal junction of cutaneous papilloma and fibropapilloma (Figure 5), exhibiting a pattern similar to those verified in immunodetection of STAT3 (Figure 4). However, it was not observed the expression of Oct-3/4 in the esophageal carcinoma (Figure 5). Although controversial, this result can be attributed to the high mitogenic activity verified in the esophageal carcinoma, verified by the presence of mitotic cells (Figure 5). Moreover, this data reinforces that the main alterations verified in the tumor microenvironment occur from a pre-malignant stage.
The CSC phenotype acquisition confers resistance to apoptosis in cells genetically unstable. In this context, the BPV E6-mediated p53 downregulation confers an additional anti-apoptotic action, at the same time that induce DNA damages 91. These combined action contribute with both cancer initiation, increasing the entropic status 33 and, metastasis, conferring anoikis resistance, which is recognized as a EMT hallmark 92. On the one hand the CSC phenotype acquisition is mandatory to distant metastasis formation, on the other hand, it represents a challenge for therapeutics.
Added to these results, we verified the immunodetection of vimentin (VIM) in dermis in all tissues analyzed (Figure 6). As expected the samples of papilloma 02 and 03 (fibropapilloma) showed an expressive expression of VIM into the dermis, confirming the BPV fibrotropism described in the histopathological analysis (Figure 1). However, it was also verified the VIM expression in keratinocytes present in both dermo-epidermal junction and subrabasal epithelium layer (Figure 6). The VIM overexpression has been reported in HPV-related head and neck squamous cell 93, 94, cervical 22 and penile carcinomas 95.
Vimentin is one of the most abundant mesenchymal protein expressed in mammals 96. The VIM is evolutionary conserved 20, for this reason, the anti-vimentin clone V9 antibody has been used for the vimentin detection in bovines 97, 98. The protein confers mechanic-structural support for mesenchymal cells 99 and has multiple phosphorilation sites 20, which act as substrate for different kinases, including the Rho kinases 99, 100. The phosphorilation of these sites leads to VIM depolymerization, confering cell motility 99. Due to these reasons, the overexpression of VIM is considered a canonical marker of EMT 20.
However, the alterations that lead to EMT are not restrict to cell genetic and epigenetic deregulations. Changes in the extracellular matrix (ECM) are also mandatory to allow the transformed cell migration. For this reason, we also analyzed the collagen composition of ECM using the Picrosirius red staining, method developed by Constantine and Mowry in 1968 101. This method allows to identify collagen fibers of type I and II 27, which are crucial for the tissue integrity maintenance 102. Under a wave-lenght of 540 nm, collagen type I fibers are displayed in red-orange color, while fibers of type III, yellow-green 27.
Results of this analysis showed a significant reduction in collagen type III fibers and a consecutive increase in type I fibers in samples from cutaneous papilloma, fibropapillomas and esophageal carcinoma in relation to the BPV-free normal skin (Figure 7). These data reinforces that the BPV can promote the ECM remodeling, leading to EMT. This because the collagen type I fibers can activate focal adhesion kinase (FAK), resulting in the E-cadherin dissociation, that is recognized as a EMT hallmark 103, 104. This process results in the nuclear translocation of β-catenin, promoting the E-cadherin downregulation 105.
In summary, these combined data reinforce our previous results using cell cultures, validating both systems (cell culture and paraffin-embed tissues) as useful models to study the natural history of BPV-infected lesions. Altogether, the results from these systems indicate that the BPV promote the cancer progression and metastasis through the transdifferentiation of an epithelial to mesenchymal cells (EMT).
Acknowledgments
The authors thank the collaboration with Prof. Dr. Franco Roperto (University of Naples Federico II) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant number 2014/20617-5) by the financial support.
References
- 1.RC Stocco dos Santos, CJ Lindsey, OP Ferraz, JR Pinto, RS Mirandola et al. (1998) Bovine papillomavirus transmission and chromosomal aberrations: an experimental model. , J. Gen. Virol 7, 2127-35.
- 2.Araldi R, Assaf S, Carvalho R, Carvalho M, Mazzuchelli-de-Souza J et al. (2017) Papillomaviruses a systematic review. , Genet. Mol. Biol 1.
- 3.Campos S, Melo T, Assaf S, Araldi R, Mazzuchelli-de-Souza J et al. (2013) Chromosome aberrations in cells infected with bovine papillomavirus: comparing cutaneous papilloma, esophagus papilloma, and urinary bladder lesion cells. ISRN Oncol. 910849.
- 4.Cota J, Peleteiro M, Petti L, Tavares L, Duarte A. (2015) Detection and quantification of bovine papillomavirus type 2 in urinary bladders and lymph nodes in cases of bovine enzootic hematuria from the endemic region of Azores. , Vet. Microbiol 178(1), 138-43.
- 5.RF Carvalho, RP Araldi, TAN Lima, DG Modolo, JM Souza et al. (2016) Synergic associations between the bovine papillomavirus infection and alimentary cofactors. , Ref. Modul. Food. Sci 1, 1-10.
- 6.TC Melo, Diniz N, SRC Campos, OP Ferraz, CJ Lindsey et al. (2011) Cytogenetic studies in peripheral blood of bovines afflicted by papillomatosis. , Vet. Comp. Oncol 9(4), 269-74.
- 7.Araldi R, Melo T, Diniz N, Carvalho R, Beçak W et al. (2013) Bovine papillomavirus clastogenic effect analyzed in comet assay. Biomed Res. Int. 1-7.
- 8.Araldi R. (2015) Bovine papillomavirus: What we know and what we should know. Araldi R, editor. Lambert 124, p..
- 9.Araldi R, Melo T, Neves A, Spadacci-Morena D, Magnelli R et al. (2015) Hyperproliferative action of bovine papillomavirus (BPV): Genetics and histopathological aspects. , Genet. Mol. Res 14(4), 12942-54.
- 10.Araldi R, Módolo D, De-Sá-Júnior P, Consonni S, Carvalho R et al. (2016) Genetics and metabolic deregulation following cancer initiation: A world to explore. , Biomed. Pharmacother 82, 449-58.
- 11.Campos S, Trindade C, Ferraz O, Giovanni D, Lima A et al. (2008) Can established cultured papilloma cells harbor bovine papillomavirus?. , Genet. Mol. Res 7(4), 1119-26.
- 12.Araldi R, De-Sá-Júnior P, Magnelli R, Modolo D, Mazzuchelli-de-Souza J et al. (2016) Primary cultures derived from bovine papillomavirus-infected lesions as model to study metabolic deregulation. , J. Cancer Res. Ther. Oncol 4(103), 1-18.
- 13.Simões R, Barth O. (2016) Chromosome aberrations as a biomarker for genomic instability in cell cultures originated from bovines, canines and equines infected with papillomavirus. , Int. J. Appl. Sci. Biotechnol 4(1), 104-12.
- 14.RP Araldi, De Melo TC, SR Consonni, JM Souza, Grando D et al. (2017) Bovine papillomavirus productive infection in cell cultures First evidences. , Virol. Res 1(2).
- 15.Ferguson S, Srinivasan V, Heimberger A. (2015) The role of STAT3 in tumor-mediated immune suppression. , J. Neurooncol 1.
- 16.Lin L, Fuchs L, Li C, Olson V, Bekaii-Saab T et al. (2011) STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH +/CD133 + stem cell-like human colon cancer cells. , Biochem. Biophys. Res. Commun 416(3), 246-51.
- 17.Nguyen K, Zong C, Uttamsingh S, Sachdev P, Bhanot M et al. (2002) The role of phosphatidylinositol 3-kinase, Rho family GTPases, and STAT3 in Ros-induced cell transformation. , J. Biol. Chem 277(13), 11107-15.
- 18.Terui K, Enosawa S, Haga S, Zhang H, Kuroda H et al. (2004) Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. , J. Hepathology 41, 957-65.
- 19.Trusolino L, Bertotti A, Comoglio P. (2010) MET signalling: principles and functions in development, organ regeneration and cancer. , Nat. Ver. - Cancer 11(12), 834-48.
- 20.Satelli A, Li S. (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 68(18), 3033-46.
- 21.Araldi R, Lima T, Módolo D, Mazzuchelli-de-Souza J, Magnelli R et al. (2017) Analysis of stem-cell and migratory phenotype in primary cultures derived from BPV-infected benign and malignant neoplasms. , J. Cancer Res. Ther. Oncol 5(101), 1-13.
- 22.Qureshi R, Arora H, Rizvi M. (2015) EMT in cervical cancer: Its role in tumour progression and response to therapy. , Cancer Lett 356(2), 321-31.
- 23.Chang L, Graham P, Hao J, Ni J, Bucci J et al. (2013) Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Deas. 4, 875.
- 24.Radisky D, LaBarge M. (2008) Epithelial-mesenchymal transition and the stem cell phenotype. , Cell Stem Cell 2(6), 511-2.
- 25.SA Mani, Guo W, MJ Liao, EN Eaton, Ayyanan A et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. , Cell 133(4), 704-15.
- 26.Singh A, Settleman J. (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. , Oncogene 29(34), 4741-51.
- 27.Ribeiro F, Carvalho M, Pereira C, Tateno D. (2015) Análise da concentração de colágeno tipo I e III presente no reparo de feridas tratadas com mitomicina C em ratos. Arq. Médicos Hosp. Fac. Ciências Médicas da St. Casa São Paulo 60(11), 22-6.
- 28.Hadi A, Mouchaers K, Schalij I, Grunberg K, Meijer G et al. (2011) Rapid quantification of myocardial fibrosis: A new macro-based automated analysis. , Cell Oncol 34(4), 343-54.
- 29.Morel A, Lièvre M, Thomas C, Hinkal G, Ansieau S et al. (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 3(8), 2888.
- 30.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. (2005) Migrating cancer stem cells - an integrated concept of malignant tumour progression. , Nat. Ver. Cancer 5(9), 744-9.
- 32.Clevers H. (2011) The cancer stem cell: premises, promises and challenges. , Nat. Med 17(3), 313-9.
- 33.Csermely P, Hódsági J, Korcsmáros T, Módos D, Perez-Lopez A et al. (2014) Cancer stem cells display extremely large evolvability: alternating plastic and rigid networks as a potential Mechanism. Network models, novel therapeutic target strategies, and the contributions of hypoxia, inflammation and cellular senescence. , Semin. Cancer Biol 30, 42-51.
- 34.PB Gupta, CL Chaffer, RA Weinberg. (2009) Cancer stem cells: mirage or reality?. , Nat. Med 15(9), 1010-2.
- 35.Visvader J, Lindeman G. (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. , Nat. Ver 8(10), 755-68.
- 37.Negrini S, Gorgoulis V, Halazonetis T. (2010) Genomic instability-an evolving hallmark of cancer. , Nat. Rev 11(3), 220-8.
- 38.Denisenko T, Sorokina I, Gogvadze V, Zhivotovsky B. (2016) Mitotic catastrophe and cancer drug resistance: a link that must to be broken. , Drug Resist Updat 24, 1-12.
- 39.Liang Y, Zhong Z, Huang Y, Deng W, Cao J et al. (2010) Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. , J. Biol. Chem 285(7), 4931-40.
- 40.Melo T, Araldi R, Pessoa N, De-Sá-Júnior P, Carvalho R et al. (2015) Bos taurus papillomavirus activity in peripheral blood mononuclear cells demonstrating a productive infection. , Genet. Mol. Res 14(4), 16712-27.
- 42.Borzacchiello G, Resendes A, Roperto S, Roperto F. (2009) Co-expression of bovine papillomavirus E5 and E7 oncoproteins in naturally occurring carcinomas of the urinary bladder in cattle. , J. Comp. Pathol 141(1), 84-8.
- 43.Russo V, Roperto F, Esposito I, Ceccarelli D, Zizzo N et al. (2016) ERas protein is overexpressed and binds to the activated platelet-derived growth factor β receptor in bovine urothelial tumour cells associated with papillomavirus infection. from: http://linkinghub.elsevier.com/retrieve/pii/S1090023316000058 , Vet. J. Available
- 44.Potocki L, Lewinska A, Klukowska-Rötzler J, Bielak-zmijewska A, Grabowska W et al. (2014) Sarcoid-derived fibroblasts: links between genomic instability, energy metabolism and senescence. , Biochimie 92, 163-72.
- 45.Matoltsy A, Matoltsy M. (1970) The chemical nature of keratohyalin granules of the epidermis. , J. Cell Biol 47, 593-603.
- 47.Fletcher S. (1983) Histopathology of papilloma virus infection of the cervix uteri the history, taxonomy, nomenclature and reporting of koilocytic dysplasias. , J. Clin. Pathol 36, 616-24.
- 48.Ferraro C, Canedo M, Oliveira S, Carvalho M, Dias E. (2011) Infecção oral pelo HPV e lesões epiteliais proliferativas associadas. , J. Bras. Patol. Médica e Lab 47(4), 451-9.
- 49.Heng B, Glenn W, Ye Y, Tran B, Delprado W et al. (2009) Human papilloma virus is associated with breast cancer. , Braz. J. Cancer 101, 1345-50.
- 50.Monte T, Peixoto G. (2010) A incidência de papilomavírus humano em mulheres no Hospital Universitário Sul Fluminense. , Ver. Bras. Análises Clínicas 42(2), 131-9.
- 51.Betiol J, Kignel S, Trsitão W, Arruda A, Santos A et al. (2012) HPV-18 prevalence in oral mucosa diagnosed with verrucous leukoplakia: cytological and molecular analysis. , J. Clin. Pathol 65(8), 2012-4.
- 52.Rogovskyy A, Baszler T, Bradway D, Bruning D, Davitt C et al. (2012) A novel papillomavirus isolated from proliferative skin lesions of a wild American beaver (Castor canadensis). , J. Vet. Diagnostic Investig 24(4), 750-4.
- 53.Krawczyk E, Suprynowicz F, Liu X, Dai Y, Hartmann D et al. (2008) Koilocytosis: a cooperative interaction between the human papillomavirus. , Am. J. Pathol 173(3), 682-8.
- 54.Löhrdr C, Juan-sallésd C, Rosas-Rosas A, García A, Garnerd M et al. (2005) Sarcoids in captive zebras (Equus burchellii): association with bovine papillomavirus type 1 infection. , J. Zoo Wild Med 36, 74-81.
- 55.Lunardi M, Alcântara B, Otonel R, Rodrigues W, Alfieri A et al. (2013) Bovine papillomavirus type 13 DNA in equine sarcoids. , J. Clin. Microbiol 51(7), 2167-71.
- 56.Schulman F, Krafft A, Janczewski T. (2001) Feline cutaneous fibropapillomas: Clinicopathologic findings and association with papillomavirus infection. , Vet. Pathol 38(3), 291-6.
- 57.Teifke J, Kidney B, Löhr C, Yager J. (2003) Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. , Vet. Dermatol 14(1), 47-56.
- 58.Alberti A, Pirino S, Pintore F, MF Addis, Chessa B et al. (2010) Ovis aries Papillomavirus 3: a prototype of a novel genus in the family Papillomaviridae associated with ovine squamous cell carcinoma. , Virology 407(2), 352-9.
- 60.Elzein E, Sundberg J, Housawi F, Gameel A, Ramadan R et al. (1991) Genital bovine papillomavirus infection in Saudi Arabia. , J. Vet. Diagnostic Investig 3(1), 36-8.
- 61.Schulman F, Krafft A, Janczewski T, Reupert R, Jackson K et al. (2003) Camelid mucoutaneous fibropapillomas: clinicopathologic findings and association with papillomavirus. , Vet. Pathol 40, 103-7.
- 62.Dyk E van, Bosman A, Wilpe E van, Willians J, Bengis R et al. (2011) Detection and characterisation of papillomavirus in skin lesions of giraffe and sable antelope in South Africa. , J. South Africa Veterninary Assiciation 82(2), 80-5.
- 65.Tomita Y, Ogawa T, Jin Z, Shirasawa H. (2007) Genus specific features of bovine papillomavirus E6, E7, E5 and E8 proteins. Virus Res. 124(1), 231-6.
- 66.Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S et al. (2011) Papillomavirus E5 the smallest oncoprotein with many functions. , Mol. Cancer 10(1), 140.
- 67.Costa R, Medeiros R. (2014) Bovine papillomavirus: opening new trends for comparative pathology. , Arch. Virol. [Internet] 159(2).
- 68.Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A et al. (2013) Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. , Neurology 80(2), 181-7.
- 69.Chambers G, Ellsmore V, O’Brien P, Reid S, Love S et al. (2003) Sequence variants of bovine papillomavirus E5 detected in equine sarcoids. Virus Res. 96(1), 141-5.
- 70.Borzacchiello G. (2007) Bovine papillomavirus infections in animals. , Commun Curr. Res. Educ. Top Trends Appl. Microbiol 673-9.
- 71.Rampias T, Sasaki C, Psyrri A. (2013) Molecular mechanisms of HPV induced carcinogenesis in head and neck. , Oral Oncol 50(5), 356-63.
- 72.Buchanan D, Kurita T, Taylor J, Lubahn D, Cunha G et al. (1998) Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. , Endocrinology 139(10), 4345-52.
- 73.Carrilho C, Alberto M, Buane L, David.L.(2004): Keratins 8, 10, 13, and 17 are useful markers in the diagnosis of human cervix carcinomas. , J Hum Pathol 35(5), 546-51.
- 74.Maddox P, Szarewski A, Dyson J, Cuzick J. (1994) Cytokeratin expression and acetowhite change in cervical epithelium. , J. Clin. Pathol 47, 15-7.
- 75.Barak V, Goike H, Panaretakis K, Einarsson R. (2004) Clinical utility of cytokeratins as tumor markers. , Clin. Biochem 37, 529-40.
- 76.Pérez-Martínez C, García-Fernández R, Escudero A, Ferreras M, García-Iglesias M. (2001) Expression of cytokeratins and vimentin in normal and neoplastic tissue from the bovine female reproductive tract. , J. Comp. Pathol 124, 70-8.
- 77.Yang X, Xu Y, Shi G, Fan J, Zhou J et al. (2008) Cytokeratin 10 and cytokeratin 19: Predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. , Clin. Cancer Res 14(12), 3850-9.
- 78.Zavizion B, Duffelen M van, Schaeffer W, Politis I. (1996) Establishment and characterization of a bovine mammary myoepithelial cell line. , In vitro Cell Dev. Biol 32, 149-58.
- 79.Moll R, Divo M, Langbein L. (2008) The human keratins: Biology and pathology. , Histochem Cell Biol 129(6), 705-33.
- 80.Rieger M, Franke W. (1988) Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. , J. Mol. Biol 204(4), 841-56.
- 81.Shukla S, Shishodia G, Mahata S, Hedau S, Pandey A et al. (2010) Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: implications in high-risk human papillomavirus infection. , Mol. Cancer 9(1), 282.
- 82.Sriuranpong V, Park J, Amornphimoltham P, Patel V, Nelkin B et al. (2003) Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. , Cancer Res 63, 2948-56.
- 84.Yamaoka K, Saharinen P, Pesu M, Holt V, Silvennoinen O et al. (2004) The Janus kinases (Jaks). , Genome Biol 5(12), 253.
- 85.Fofaria N, Srivastava S. (2014) STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. , Carcinogenesis 36(1), 142-50.
- 86.Chen H, Hutt-fletcher L, Cao L, SD Hayward. (2003) A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. , J. Virol 77(7), 4139-48.
- 87.Johnston P, Grandis J. (2011) STAT3 signaling: anticancer strategies and challenges. , Mol. Inerventions 11(1), 18-26.
- 89.Williams V, Brichler S, Khan E, Chami M, Dény P et al. (2012) Large hepatitis delta antigen activates STAT-3 and NF-kB via oxidative stress. , J. Viral Hepat 19, 744-53.
- 90.Argyle D, Blacking T. (2008) From viruses to cancer stem cells: Dissecting the pathways to malignancy. , Vet. J 177(3), 311-23.
- 91.Araldi R, Mazzuchelli-de-souza J, Modolo D, Souza E, Melo T et al. (2015) Mutagenic potential of Bos taurus papillomavirus type 1 E6 recombinant protein First description. Biomed Res. Int
- 92.Massagué J, Obenauf A. (2010) Metastatic colonization by circulating tumor cells. , Science 5(1).
- 93.Bayo P, Jou A, Stenzinger A, Shao C, Gross M et al. (2015) Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. , Mol. Oncol 9(8), 1704-19.
- 94.Pitiyage G, Lei M, Guererro-Urbano T, Odell E, Thavaraj S. (2015) Biphenotypic human papillomavirus-associated head and neck squamous cell carcinoma: a report of two cases. , Diagn. Pathol 10(1), 97.
- 95.Masferrer E, Ferrándiz-Pulido C, Masferrer-Niubò M, Rodríguez-Rodríguez A, Gil I et al. (2015) Epithelial-to-mesenchymal transition in penile squamous cell carcinoma. , J. Urol 193(2), 699-705.
- 96.Castro-Muñozledo F, Velez-DelValle C, Marsch-Moreno M, Hernández-Quintero M, Kuri-Harcuch W. (2014) Vimentin is necessary for colony growth of human diploid keratinocytes. , Histochem. Cell. Biol 143(1), 45-57.
- 97.Rossi B, Merlo B, Colleoni S, Iacono E, PL Tazzari et al. (2014) Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. , Stem Cell Ver 10, 712-24.
- 98.Martano M, Corteggio A, Restucci B, Biase M, Borzacchiello G et al. (2016) Extracellular matrix remodeling in equine sarcoid: an immunohistochemical and molecular study. , BMC Vet. Res 12(1), 24.
- 99.Lahat Q, Zhu Q, Huang K, Wang S, Bolshakov S et al. (2010) Vimentin is a novel anti-cancer therapeutic target; insights from In Vitro and In Vivo mice xenograft studies. , PLoS One 5(4).
- 100.Huang C, Devanney J, Kennedy S. (1988) Vimentin, a cytoskeletal substrate of protein kinase. , C. Biochem. Biophys. Res. Commun 150(3), 1006-11.
- 101.Dayan D, Hiss Y, Hirshberg A, JJ Bubis, Wolman M. (1989) Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers?. , Histochemistry 93(1), 27-9.
- 102.Rich L, Whittaker P. (2005) Collagen and Picrosirius red staining a polarized light assessment of fibrillar hue and spatial distribution. , Brazilian J. Microbiol 22(2), 97-104.
- 103.Koenig A, Mueller C, Hasel C, Adler G, Menke A. (2006) Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 66(9), 4662-71.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
Vitor Rodrigues da Costa, O. F. Souza, Michelli Ramires Teixeira, A. Alievi, H. Vigerelli et al. - Exploration of Immunology (2023) Semantic Scholar
Exploration of Immunology (2023) OpenAlex
Exploration of Immunology (2023) Crossref
