Anti-Oxidant Phytochemicals as Potential Treatments for Age-Related Macular Degeneration
Abstract
Age-related macular degeneration (AMD) is responsible for a substantial proportion of severe visual impairment and blindness in people over 50 years of age. Current treatments for AMD are not effective in all patients and a proportion of patients who respond well to the treatment will still eventually develop central visual impairment. Despite all efforts to develop safe and efficient medications for AMD, as yet pharmacological approaches have failed to provide fully effective treatments for this condition. Various lines of evidence attest to the contributions of oxidative stress in the etiology of AMD. Anti-oxidant nutrients may be valuable preventive or therapeutic agents however complementary therapies can become widely adopted without sufficient knowledge of the real advantages and liabilities. This review considers the interventional potential of some common phytochemicals for treating AMD, primarily focusing on clinical and epidemiological evidence of potential public health relevance.
Author Contributions
Academic Editor: Suowen Xu, University of Rochester
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 A. Shahandeh, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Age-related macular degeneration (AMD) is a disease of the elderly, especially those of European ancestry, in which there is irreversible central vision loss and ultimately blindness 1, 2, 3. As lifespans increase, AMD poses an increasingly serious issue for the aging population 4 and is now a well-recognized public health problem with substantial social and economic impact 5. Epidemiological studies have identified AMD as the leading cause of blindness in the demographic of individuals 55 years and over in industrialized countries 6, 7, 8. There is a strong need for greater understanding of the underlying pathology and molecular mechanisms to guide the development of effective therapies.
As shown in Figure 1, the macula, from the Latin macula lutea, macula meaning spot and lutea yellow, is located near the centre of retina. The pathological processes in AMD lead to damage and degeneration of photoreceptors within this region 9, causing impaired sight in the centre of the visual field.
Figure 1.Cross section of the eye. The macula is located in the posterior segment of the eye. The arrangement of retinal layers is shown in the enlarged inset on the right. The neural retina is isolated from the blood stream (choroidal vessels) by the blood-retina barrier, formed by the retinal pigment epithelium (RPE). Light travels through the different layers to strike the rod and cone cells (photoreceptors) and start the visual cycle.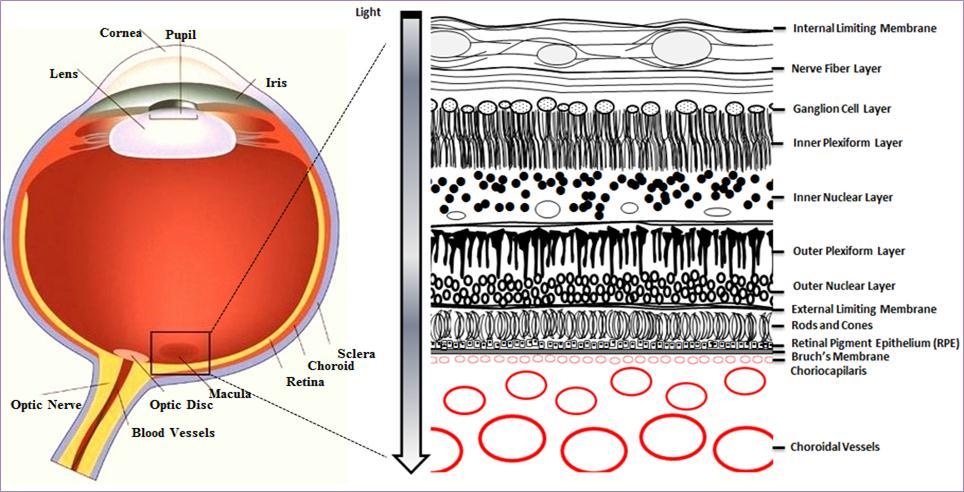
AMD can be divided into two stages: early AMD, where there is mild pathology but no obvious vision abnormalities, and late AMD, which manifests as severe vision loss 10. The pathology of early AMD is characterised by drusen, extracellular deposits of cellular debris between the retinal pigment epithelium (RPE) and Bruch’s membrane (Figure 1) that range in colour from yellow to white under ophthalmoscopy 11. While early AMD itself does not manifest as noticeable vision loss in affected individuals, it is associated with a significant risk of progression to late AMD, where substantial loss of visual function is often reported 9. Late AMD is classified into two forms, wet and dry, based on whether there is choroidal neovascularisation. Both forms have drusen but whereas dry AMD is non-neovascular and characterised by geographic atrophy that extends to the centre of the macula, in contrast wet AMD is characterised by choroidal neovascularisation and increased vessel fragility and permeability 12.
While the etiology and pathophysiology of AMD are still incompletely defined, it has been proposed that oxidative processes play a contributory role 13, 14. Although a wide array of clinical and experimental drugs using anti-oxidant components is currently applied in degenerative diseases 15, it is hypothesized that there are certain diet-based compounds with the capacity to strengthen defence systems in response to oxidative stress and inflammation 16, 17. In fact, the potential benefits of nutrient supplementation for age-related eye diseases have been recognized for some time 18. This review presents evidence for putative roles of natural dietary anti-oxidants as preventative or interventional compounds in AMD.
Oxidative Stress in the Etiology of AMD
Generation of reactive oxygen species (ROS) and other cellular oxidants is part of normal cellular metabolism. Although low to moderate concentrations of ROS, in combination with lysosomal digestive enzymes, can contribute to immune system defences against invading pathogens or apoptotic cells, overproduction of ROS leads to a deleterious process termed oxidative stress in which cell structures can be potentially damaged. Toxic levels of ROS are produced as a consequence of dysregulation of pro-oxidant and anti-oxidant homeostasis 19.
The retina, where high oxygen consumption occurs in conjunction with light exposure, is extremely vulnerable to high concentrations of ROS, which are associated with lipid peroxidation, DNA and protein damage 16, 20, 21. The retinal pigment epithelium (RPE) and neural retina (Figure 1) are partly protected against high concentrations of ROS by both enzymatic and non-enzymatic anti-oxidants 16 however accumulation of pro-oxidants over time, and the resulting increases in ROS, are associated with the development of AMD 22, 23.
The blood-retina barrier partly isolates the retina from the bloodstream and restricts the access of many medications to the retina, a complicating factor for treatments targeting retinal disease. In contrast, there is a broad range of biologically active compounds in plants known as phytochemicals these are able to cross this barrier 24, 25. Increasing evidence suggests that phytochemicals might be valuable in treating AMD, in addition to other age-related degenerative disorders such as Alzheimer’s disease and Parkinson’s disease 26, 27, 28, 29.
Dietary Interventions
The beneficial effects of daily consumption of phytochemicals or bioactive compounds have been reviewed elsewhere in the context of major diseases such as cancer 30, 31, cardiovascular disease 32, 33 and neurodegenerative disease 34, 35. Due to their anti-oxidant properties and low toxicity, natural food compounds are excellent candidates for protecting against degeneration and other age-related problems (‘anti-aging’). Phytochemicals have many double bonds in their molecular structures which, by reacting with ROS, enable them to scavenge free radicals. In addition to potential actions in preserving neural function and forestalling age-related deficits, the ideal characteristic of phytochemicals in regard to the treatment and prevention of AMD is their ability to pass through the blood-retina barrier. The following sections review a number of phytochemicals and their effects on AMD.
Carotenoids
Carotenoids are powerful antioxidants and considered to be the main singlet oxygen quenchers in chloroplasts 36. One important early study was a large multicentre case-control study 18, which reported an inverse association between advanced AMD and levels of carotenoid intake. Using multiple logistic-regression analyses, the authors estimated the relative risk for AMD based on dietary indicators of antioxidant status. Their findings showed consumption of carotenoids, especially lutein and zeaxanthin, substantially decrease the risk for AMD 18. Rich dietary sources for lutein and zeaxanthin include spinach and collard greens. These carotenoids are described in more detail below.
The initial Age-Related Eye Disease Study (AREDS), a large-scale clinical trial with 3 650 participants (55-80 years) spanning over six years, evaluated the effects on AMD progression of various dietary supplements, including beta-carotene, vitamin C, vitamin E, and zinc 38. Randomly selected subsets of participants received either placebo or a daily antioxidant tablet containing beta carotene (15 mg), vitamin E (400 IU), vitamin C (500 mg), zinc oxide (80 mg) and cupric oxide (2 mg). Participants receiving the supplement had reduced rates of progression of advanced AMD compared to those receiving the placebo, with significantly smaller drusen deposits. However the AREDS formulation examined in these studies was representative of pharmacological dosages used clinically and is too rich in anti-oxidants to be readily achievable even for people on common daily multivitamin supplementation regimens 37.
One serious limitation of beta-carotene use is its association with an increased risk of lung cancer in smokers, which was first reported after the commencement of AREDS 38. This compelled researchers to look for substitutes for beta-carotene.
The follow-up Age-Related Eye Disease Study 2 (AREDS2) aimed to improve on the original AREDS anti-oxidant formulation for AMD treatment 38 by replacing beta-carotene with lutein and zeaxanthin 39, 40. Lutein and zeaxanthin are carotenoids which are naturally expressed in the macula, with lutein believed to be important in the peripheral macula and zeaxanthin in the central macula 41. Lutein and zeaxanthin are isomers with identical chemical formulas, differing only in the location of the double bond in one end ring 42, 43.
A self-administered, semi-quantitative, food frequency questionnaire (AREDS FFQ) was applied to assess the nutrient intake of participants aged 50 to 85 years with high risk of developing AMD (n=4203). High risk was defined as either bilateral drusen or large drusen in one eye and advanced AMD in the other eye. Over a five year period, a randomly selected subset of participants received daily dietary supplementation consisting of the carotenoids lutein (10 mg) and zeaxanthin (2 mg) in addition to the standard AREDS formulation, which included Vitamins C and E, the omega-3 fatty acids docosahexaenoic acid and eicosapentaenoic acid, copper, zinc and beta-carotene (non-smokers only). The control group received the formulation used in the original AREDS study. In total, 3451 eyes were assessed for the treatment group compared to 3440 eyes for the control group. The high lutein or zeaxanthin diet significantly lowered the chance of developing advanced AMD [hazard ratio 0.91, 95% CI (0.82-1.00), p=0.05] over the study period, although the size of the effect was not large 39, 40. Similar results were obtained in the Carotenoids in Age-Related Eye Disease Study (CAREDS). More than 1700 women aged between 50 and 79 years were followed over periods of 4 to 7 years. The researchers reported a significant risk reduction of AMD amongst women aged over 75 with a stable dietary intake of lutein and zeaxanthin which was quantified to be 2.6 mg per day 44. A schematic illustrating the structure and effect of lutein is shown in Figure 2.
Figure 2.Schematic depicting the protective action of phytochemicals on light-induced photoreceptor damage. Lutein and its isomer zeaxanthine are natural carotenoid phytochemicals present within the macula which are hypothesized to mitigate light-induced oxidative damage by scavenging of ROS.
In contrast, there have been a number of studies which have failed to observe comparable effects. For instance, in the Blue Mountains Eye Study, a large population-based assessment of visual impairment conducted in Australia 45, 2335 participants aged 49 years or older, with no trace of early age-related maculopathy, were studied over 5 years. Nutrient intakes were assessed by FFQ. No protective effects of lutein and zeaxanthin were reported in the 8.7% of participants in whom early AMD was detected by the end of the study. However most participants in this study had lower lutein and zeaxanthin intakes than intake levels reported to be associated with retinal protection of over 1.5 mg per day 39, 44, 46. It is also possible that other phytochemicals may have masked potential effects.
In another large prospective study, 118 428 participants aged over 50 years, with no baseline diagnosis of AMD, were followed for more than a decade. While the intake levels were within the range of levels previously reported to be associated with retinal protection 39, 44, 46, with median intakes of lutein/zeaxanthin ranging from 1.5 mg per day for the first quintile to 6.6 mg per day for the fifth quintile, the results reflected no strong association between dietary lutein, zeaxanthin and the risk of age-related macular disorders 47. However again the amount of lutein intake in this study was far lower than the daily dose of 10 mg of lutein used in AREDS2 39, 40.
While several reports provide evidence for protective effects of carotenoids, particularly lutein and zeaxanthin in populations at risk of AMD, further clinical studies are required to determine the optimal dosage of these phytochemicals.
Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-2,5-dione), derived from the yellow curry spice turmeric, possesses potent antioxidant properties, as reviewed previously 48, 49. Results from animal models suggest that it may have protective effects in the retina. Although curcumin has poor oral bioavailability, which may limit its therapeutic utility in the retina, it has been reported that bioavailability can be increased by encapsulating curcumin in liposomes 50, 51.
Protective effects of curcumin have been reported in rats with diabetes-induced oxidative stress and inflammation in the retina 24, supporting other studies suggesting that curcumin is able to cross the blood-retina barrier 52 and may assist the anti-oxidant defence system by scavenging free radicals. Similarly, protective pre-conditioning effects have been reported in a rat model of light-induced retinal degeneration 53. Wistar albino rats exposed to damaging light after two weeks of curcumin supplemented diet (0.2%) were found to be protected against light-induced retinal damage, as assessed by both morphological and functional measures. This appears to be mediated by changes in a number of proteins, including NF-κB and various growth factors, which result in inhibition of cellular inflammatory responses. The results suggest curcumin helps preserve the functional and structural integrity of retinal photoreceptor cells through the regulation of inflammation and oxidative stress 53.
As far as we have been able to determine, there has been no human study investigating the role of curcumin in prevention, delay or treatment of AMD. Further studies will be needed to assess the effectiveness of curcumin supplementation in preventing or treating AMD.
Ginkgo Biloba
Therapeutic applications of Ginkgo biloba can be traced back 5000 years in China and EGb 761, a standardized extract of dried leaves of Ginkgo biloba, is now being used in the treatment of various conditions, for example cerebral insufficiency 54. (Cerebral insufficiency is the term used by some researchers to refer to non-specific and poorly characterized decline in memory and other cognitive functions that may occur with aging; this should not be confused with cerebrovascular insufficiency, since clear vascular disease is not invariably present 54). With regard to the visual system, a Cochrane systematic review has analysed two randomized trials conducted on small groups of people with AMD symptoms at baseline. In one of these studies, 10 participants received Ginkgo biloba extract EGb 761 (80 mg) twice a day for six months and another 10 participants received a placebo. The other study of 99 participants compared two different doses of EGb 761 extract, 240 mg per day (n=50) and 60 mg per day (n=49), both again over a six month period 55. Although trends towards improvements in acuity, near vision and other measures were reported, these failed to reach significance. The short duration and small number of participants prevents any conclusions being drawn from these studies and further research is needed to determine the interventional utility of Ginkgo biloba for AMD.
Anthocyanins
Anthocyanins are a versatile group of vacuolar pigments with potent antioxidants qualities. They are found at high levels in certain types of berries, such as cranberries and blueberries, and a variety of other foods that are deep red, blue or purple in colour, such as eggplant. Anthocyanins have been demonstrated to have various protective properties in studies of the effects of visible and ultraviolet light stress, heat stress and drought on plants, as reviewed previously 56. Interestingly, the Chinese wolfberry, which is high in anthocyanin, has been considered for centuries to be beneficial for the eyes and is used to treat eye conditions in traditional Chinese medicine, as reviewed elsewhere 57.
The distributions of intact anthocyanin species in the eye have been evaluated in rabbit and rat models after intraperitoneal, intravenous or oral administration of blackcurrant preparations 25. The results provide evidence that blackcurrant anthocyanins enter various ocular tissues, including the retina, after IV injection or oral administration, permeating the blood-retina barrier and the blood-aqueous barrier formed by the ciliary body epithelium. Similar results were reported by Kalt and colleagues after examination of anthocyanin deposition in tissues of blueberry-fed pigs 58.
Anthocyanins from a range of sources appear to have retinoprotective properties but in most cases the mechanisms have not yet been discovered. However anthocyanins from black rice are reported to prevent retinal photochemical damage through effects involving the AP-1/NF-κB pathway leading to reduced levels of caspase 1 59. As noted above, curcumin may also affect this pathway 53.
Based on the known anti-oxidant properties of anthocyanins and observations of their capacity to cross the retinal barrier systems, there has been considerable speculation about the preventative capabilities of anthocyanins in AMD 60, 61. However, as yet, this has not been explored sufficiently in clinical and laboratory studies to validate the usage of berry extracts in AMD.
Saffron
Crocus sativus L. (saffron) is a perennial plant commonly used as a spice for enhancing the flavor, color and aroma of food. The coloring components of saffron are pharmacologically active and water-soluble carotenoids called crocins 62. A comparative in vitro study of a range of herbs, spices, fruits and nuts found that saffron had the strongest antioxidant content 63. The antioxidant effects of saffron are attributable to the presence of bioactive components such as crocins and crocetins 64, 65. The neuroprotective effects of saffron in the brain and retina have been investigated in various animal models. For example, in the brain, saffron is reported to increase oxygenation in the cerebral hemisphere of haemorrhaged rats 66 and protect hippocampal neurons and nigrostriatal dopaminergic neurons against degeneration in rat models of parkinsonism 67, 68.
In the retina, saffron pre-conditioning has been shown to protect against apoptotic photoreceptor cell death induced by harmful levels of exposure to bright light. Daily saffron supplementation (1mg/kg) of Sprague-Dawley rats for 6 weeks prior to 24 hours exposure to continuous bright light (1000 lux) decreased death of photoreceptor cells relative to treatment with 1mg/kg beta-carotene for 6 weeks, in conjunction with reduction of inflammatory reactions and stabilization of retinal morphology 69. Microarray analysis suggests that the effects of saffron in this rat model involve changes in numerous unknown entities, notably certain non-coding RNAs 70. Similarly, in mice, dietary supplementation with crocetin for five days (100 mg/kg/day) following bright light exposure (8000 lux for 3 h) has been reported to attenuate retinal photoreceptor damage and inner retinal dysfunction 71.
Researchers are now beginning to investigate the effects of saffron in AMD patients. Results from one pioneering study of short-term supplementation of 20 mg/day saffron for 90 days in twenty-five patients with early AMD has provided evidence for improvement in retinal flicker sensitivity in response to light over this period 72. This challenges the traditional idea that anti-oxidants are beneficial for AMD only after long-term applications 73.
A follow up to the above research evaluated the effects of saffron on the retina of AMD patients over longer periods of up to 16 months 74. Twenty-nine AMD patients were treated with 20 mg of dietary saffron per day for 12-16 months. Improvement in macular function (mean focal electroretinogram sensitivity, mean visual acuity) was observed after just three months of saffron supplementation and the changes were reported to be stable after that period of time.
Another study by Marangoni and colleagues 75 assessed the association between major risk genotypes for AMD and the effects of saffron. The study recruited 33 early AMD patients (age 51 years or more) with known AMD risk genotypes, notably polymorphisms in complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) proposed to be associated with clinical characteristics of AMD 76. Oral supplementation of 20 mg of saffron per day for 11 months was found to have positive functional effects on the retina, with improvements in focal electroretinogram amplitudes and macular sensitivity. The observed improvements were independent of patient genotype.
Although neuroprotective mechanisms of saffron are still to be worked out in full 70, these studies suggest saffron may have therapeutic value in degenerative conditions such as AMD.
Conclusions
Since the initiation and progression of AMD may be considerably influenced by exposure to oxidative factors, the anti-oxidant properties of phytochemicals make them excellent candidates for the prevention or treatment of AMD. The capacity to scavenge free radicals is proposed to be the main mechanism by which phytochemicals may protect against AMD. The functional characteristics of phytochemicals, in conjunction with their low-toxicity, commercial availability and ability to cross the blood-retina barrier, make these naturally derived substances an attractive option for combatting AMD.
In particular, consumption of anthocyanins, saffron and the carotenoids lutein and zeaxanthin by AMD patients may be beneficial. Additional quantitative studies are required to progress animal research and extend the findings to humans, including evaluating optimal dosages and durations of phytochemical administration and the effects of treatments combining several phytochemicals.
Overall, while reducing the risk of AMD by modifying consumption of dietary factors seems to be a practical preventative or interventional strategy, more research is required to provide patients and clinicians with accurate and comprehensive information to achieve optimal outcomes.
References
- 1.Congdon N. (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 122(4), 477-85.
- 2.C A Augood. (2006) Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol. 124(4), 529-35.
- 3.A R Rudnicka. (2012) Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 119(3), 571-580.
- 4.U M Schmidt-Erfurth. (2007) Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand. 85(5), 486-94.
- 5.J M Seddon. (2005) The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 123(3), 321-7.
- 6.Bunce C, Xing W, Wormald R. (2007) Causes of blind and partial sight certifications in England and. , Wales: 24(11), 1692-1699.
- 7.D S Friedman. (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 122(4), 564-72.
- 8.Mitchell P. (1995) Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 102(10), 1450-60.
- 9.Rattner A, Nathans J. (2006) Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 7(11), 860-72.
- 10.R E Hogg, Chakravarthy U. (2006) Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 25(3), 249-76.
- 11.Sarks S H. (1999) Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. , Br J Ophthalmol 83(3), 358-68.
- 12.R D Jager, W F Mieler, J W Miller. (2008) Age-related macular degeneration. , N Engl J Med 358(24), 2606-17.
- 13.Migliore L, Coppede F. (2009) Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res,674(1-2):. 73-84.
- 14.Behl C, Moosmann B. (2002) Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med. 33(2), 182-91.
- 15.Moosmann B, Behl C. (2002) Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs. 11(10), 1407-35.
- 16.Beatty S. (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 45(2), 115-34.
- 18.J M Seddon. (1994) Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 272(18), 1413-20.
- 19.K J Barnham, C L Masters, A I Bush. (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 3(3), 205-14.
- 20.Akyol O. (2002) The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry. 26(5), 995-1005.
- 21.D Y Yu, S J Cringle. (2005) Retinal degeneration and local oxygen metabolism. Exp Eye Res. 80(6), 745-51.
- 23.Khandhadia S, Lotery A. (2010) Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 12, 34.
- 24.R A Kowluru, Kanwar M. (2007) Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond). 4, 8.
- 25.Matsumoto H. (2006) Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 83(2), 348-56.
- 26.R van Leeuwen. (2005) Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 294(24), 3101-7.
- 27.J T Eells. (2008) Photobiomodulation for the treatment of retinal injury and retinal degenerative diseases. in Proceedings of Light-Activated Tissue Regeneration and Therapy Conference.Springer .
- 28.Pallas M. (2009) Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 6(1), 70-81.
- 29.Andersen J K. (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med,10Suppl:. 18-25.
- 30.Soerjomataram I. (2010) Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. , Eur J Cancer 46(14), 2563-80.
- 32.A R Ness. (2005) Diet in childhood and adult cardiovascular and all cause mortality: the Boyd Orr cohort. Heart. 91(7), 894-8.
- 33.Bhupathiraju S N, Tucker K L. (2011) Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta,412(17-18):. 1493-514.
- 34.Ebrahimi A, Schluesener H. (2012) Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 11(2), 329-45.
- 35.Kim J, H J Lee, K W Lee. (2010) Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. , J Neurochem 112(6), 1415-30.
- 36.Ramel F. (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci U S A 109(14), 5535-40.
- 37.Age-Related Eye Disease Study Research Group, A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss:. AREDS report no. 8. Arch Ophthalmol,2001 119(10), 1417-36.
- 38.G S Omenn. (1996) Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. , N Engl 334(18), 1150-5.
- 39.J P SanGiovanni. (2007) The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study:. AREDS Report No. 22. Arch Ophthalmol 125(9), 1225-32.
- 40.Age-Related Eye Disease Study 2 Research Group, Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial.JAMA,2013. 309(19):. 2005-15.
- 41.R A Bone. (1997) Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 64(2), 211-8.
- 42.Sujak A. (1999) Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys. 371(2), 301-7.
- 43.J T Landrum, R A Bone. (2001) Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 385(1), 28-40.
- 44.S M Moeller. (2006) Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 124(8), 1151-62.
- 45.Flood V. (2002) Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 109(12), 2272-8.
- 46.W G Christen. (2008) Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 126(1), 102-9.
- 47.Cho E. (2004) Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 122(6), 883-92.
- 48.Scartezzini P, Speroni E. (2000) Review on some plants of Indian traditional medicine with antioxidant activity. , J Ethnopharmacol,71(1-2): 23-43.
- 49.Suhaj M. (2006) Spice antioxidants isolation and their antiradical activity: a review. , J Food Compost Anal,19(6-7): 531-537.
- 50.R A Sharma, A J Gescher, W P Steward. (2005) Curcumin: the story so far. , Eur J Cancer 41(13), 1955-68.
- 51.Li L, Braiteh F S, Kurzrock R. (2005) Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. , Cancer 104(6), 1322-31.
- 52.Yang F. (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. , J Biol Chem 280(7), 5892-901.
- 53.Mandal M N. (2009) Curcumin protects retinal cells from light-and oxidant stress-induced cell death. , Free Radic Biol Med 46(5), 672-9.
- 54.Smith P F, Maclennan K, Darlington C L. (1996) The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). , J Ethnopharmacol 50(3), 131-9.
- 55.Evans J R. (2013) Ginkgo biloba extract for age-related macular degeneration. , Cochrane Database Syst Rev 1, 001775.
- 56.Liakopoulos G. (2006) The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). , Ann Bot 98(1), 257-65.
- 57.Wang X, Hu S. (2001) [A review of research actuality on age-related macular degeneration]. , Yan Ke Xue Bao 17(4), 245-51.
- 58.Kalt W. (2008) Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. , J Agric Food Chem 56(3), 705-12.
- 59.Jia H. (2013) Black rice anthocyanidins prevent retinal photochemical damage via involvement of the AP-1/NF-kappaB/Caspase-1 pathway in Sprague-Dawley rats. , J Vet Sci 14(3), 345-53.
- 60.Sadilova E, Carle R, Stintzing F C. (2007) Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. , Mol Nutr Food Res 51(12), 1461-71.
- 61.al Bagchi D et. (2006) Safety and whole-body antioxidant potential of a novel anthocyanin-rich formulation of edible berries. , Mol Cell Biochem,281(1-2): 197-209.
- 62.Tarantilis P A, Polissiou M, Manfait M. (1994) Separation of picrocrocin, cis-trans-crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. , J Chromatogr A 664(1), 55-61.
- 63.Pellegrini N. (2006) Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. , Mol Nutr Food Res 50(11), 1030-8.
- 64.Giaccio M. (2004) Crocetin from saffron: an active component of an ancient spice. , Crit Rev Food Sci Nutr 44(3), 155-72.
- 65.Laabich A. (2006) Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. , Invest Ophthalmol Vis Sci 47(7), 3156-63.
- 66.Seyde W C. (1986) Carotenoid compound crocetin improves cerebral oxygenation in hemorrhaged rats. , J Cereb Blood Flow Metab 6(6), 703-7.
- 67.Ahmad A S. (2005) Neuroprotection by crocetin in a hemi-parkinsonian rat model. , Pharmacol Biochem Behav 81(4), 805-13.
- 68.Abe K. (1998) Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. , Brain Res 787(1), 132-8.
- 69.Maccarone R, S Di Marco, Bisti S. (2008) Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. , Invest Ophthalmol Vis Sci 49(3), 1254-61.
- 70.Natoli R. (2010) Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. , Mol Vis 16, 1801-22.
- 71.Yamauchi M. (2011) Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. , Eur J Pharmacol 650(1), 110-9.
- 72.Falsini B. (2010) Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. , Invest Ophthalmol Vis Sci 51(12), 6118-24.
- 73.Klein M L. (2008) CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. , Ophthalmology 115(6), 1019-25.
- 74.Piccardi M. (2012) A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med,2012:. 429124.
Cited by (10)
This article has been cited by 10 scholarly works according to:
Citing Articles:
Acta Ophthalmologica (2022) Crossref
Niina Harju - Acta ophthalmologica (2022) Semantic Scholar
Acta Ophthalmologica (2022) OpenAlex
Hoda Ma, R. Liutkevičienė - Acta medica Lituanica (2021) Semantic Scholar
Oncotarget (2017) Crossref
Guojun Wu, Wen-hong Zhou, Junfeng Zhao, Xiaohua Pan, Yongjie Sun et al. - OncoTarget (2017) Semantic Scholar
Oncotarget (2017) OpenAlex
Bin Wang, Yansen Li, M. Mizu, Toma Furuta, Chunmei Li - Tissue & Cell (2017) Semantic Scholar
Oncotarget (2017) Crossref
Tissue and Cell (2016) OpenAlex
J. Yin, Miaomiao Wu, Yuying Li, W. Ren, Hao Xiao et al. - OncoTarget (2016) Semantic Scholar
