Abstract
Xanthine oxidase is a commercially important enzyme with wide area of medical applications to develop diagnostic kits. Xanthine oxidase was extracted, purified and characterized from sheep liver (SLXO). The purification procedure involved acetone precipitation and chromatography on DEAE-cellulose and Sephacryl S-300 columns. The sheep liver xanthine oxidase was homogeneously purified 31.8 folds with 3.5 U/mg specific activity and 24.1% recovery. SLXO native molecular weight was 150 kDa and on SDS-PAGE appeared as single major band of 75 kDa representing a homodimer protein. Isoelectric focusing of the purified SLXO resolved into two closely related isoforms with pI values of 5.6 and 5.8. The apparent Km for xanthine oxidase at optimum pH 7.6 was found to be 0.9 mM xanthine. FeCl2 and NiCl2 increased the activity of SLXO, while CuCl2 and ZnCl2 were found to be potent inhibitors of the purified enzyme. Allopurinol inhibits SLXO competitively with one binding site on the purified molecule and Ki value of 0.06 mM.
Author Contributions
Academic Editor: Vijay Bharti, DIHAR, DRDO, Ministry of Defence, Pin-901205, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Samir A.M. Zaahkouk, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Xanthine oxidase (XO) (EC 1.2.3.22) plays a key physiological role in the metabolism of purines that catalyzes the hydroxylation reaction of hypoxanthine to xanthine, and subsequently xanthine to uric acid 1. XO is a molybdo-flavin enzyme with a quaternary structure; each subunit contains molybdopterin, Flavin adenine dinucleotide (FAD), and iron-sulfur center 2, 3, 4. It can be found in almost all species from bacteria to human and in the various tissues of mammals 5. XO is widely used as a detection reagent for nucleotidases, purines, superoxide dismutases, adenosine deaminase, and inorganic phosphates 6. XO which is mostly found in the small intestine and liver gave rise to the idea that this enzyme could be responsible for detoxifying the body from mainly nitrogen containing polar aromatic compounds believed that even poor substrates for XO would be completely metabolized by this enzyme, considering its relatively high abundance in the human body. It plays a role in iron absorption in the small intestine, by oxidizing dietary iron from the ferrous state (Fe2+) to the ferric state (Fe3+) within the intestinal mucosa, facilitating the absorption of iron 7. It also mobilizes iron from the liver by promoting the release of iron ferritin 8. It oxidizes pteridines, heterocyclic bases and participates in the oxidation of sulphydryl (thiol) groups in glutathione, fatty acids, phospholipids, amino acids and epinephrine 9, 10, catalyzes the oxidation of aldehydes to carboxylic acids 11 and plays a role in alcohol metabolism 8.
In addition, a role for xanthine oxidase in antimicrobial defence, similar to NADPH oxidase in phagocytes is possible. It has been proposed that xanthine dehydrogenase in the endothelial cell cytoplasm is released following endothelial injury by microbes, and is converted to the oxidase form in the oxygen rich environment of blood. Xanthine oxidase can oxidize substrates in the blood, leading to the formation of oxygen derived free radicals which have been suggested to provide oxidative defence 12, 13. One of the most important xanthine oxidase competitive inhibitors is allopurinol that is a substrate analog, has similar chemical structure to hypoxanthine and used in treatment of hyperuricemia and gout 1, 14. In the present study, we report the purification and characterization of xanthine oxidase from sheep liver as a safe source for using it in medical applications especially diagnostic kit of superoxide dismutase (SOD).
Materials and Methods
Liver Materials
Six fresh sheep liver samples were obtained from different male individuals in a local slaughter-house, Cairo, Egypt and stored at -40 ˚C.
Chemicals
Xanthine sodium salt, Nitroblue tetrazolium (NBT), Phenazine methosulphate (PMS), Albumin from bovine serum (BSA), Diethylaminoethyl-cellulose (DEAE-Cellulose), Sephacryl S-300 and chemicals for electrophoresis were purchased from Sigma-Aldrich Chemical Co. The other chemicals were of analytical grade.
Assay of Xanthine Oxidase Activity
The reaction mixture of XO activity assay contains 1 ml 0.05 M Tris-HCl, pH 7.6 requiring 2 mM xanthine, 0.5 mM NBT and the xanthine oxidase solution. The reaction mixture was incubated for 5 minutes at 37 ◦C, centrifuged at 2000 rpm for 2 minutes and the absorbance was measured at 575 nm. To calculate XO units, a control reaction was done with 0.02 unit commercially available bovine milk xanthine oxidase 15.
Xanthine Oxidase Activity Staining on Polyacrylamide Gels
Activity staining of xanthine oxidase was carried out by submerging the gels in 50 mM Tris-HCl, pH 7.6, 0.5 mM xanthine, 0.25 mM nitroblue tetrazolium and 630 mM TEMED. Staining of the gels was continued till the activity bands appear on the gels 16.
Purification of Xanthine Oxidase from Sheep Liver
Preparation of Crude Extract
All of the procedures were performed at 4˚ C. 10 gm of sheep liver were minced and homogenized with 0.02 M Tris-HCI buffer, pH 7.6, containing 0.1 mM EDTA on ice and mixed with one volume of n-butanol. The mixture was kept at -20˚C overnight and centrifuged at 12000 x g for 30 min at 4˚C. The aqueous phase containing the enzyme activity was saved and designated n-butanol fraction. One volume prechilled acetone was added to the n-butanol fraction. The pellet was collected after centrifugation at 12000 x g for 30 min at 4˚C, washed three times with acetone and dried under vacuum. The acetone powder was dissolved in 0.02 M Tris-HCI buffer, pH 7.6, containing 0.1 mM EDTA and designated acetone fraction 17.
DEAE-Cellulose Column Chromatography
The acetone fraction was applied on DEAE-cellulose column (6 x 2.4 cm i.d.) equilibrated with 0.02 M Tris-HCI buffer, pH 7.6, containing 0.1 mM EDTA. The protein fractions were eluted with stepwise NaCl gradient ranging from 0 to 1 M prepared in the equilibration buffer at a flow rate of 60 ml / hour. 5 ml fractions were collected and the fractions containing XO activity were pooled and concentrated by lyophilization.
Sephacryl S-300 Column Chromatography
The concentrated DEAE –cellulose fractions containing XO activity were applied to a Sephacryl S-300 column (142 cm X 1.75 cm i.d.). The column was equilibrated and run with 0.02 M Tris-HCI buffer, pH 7.6, containing 0.1 mM EDTA at a flow rate of 30 ml / hour and 2 ml fractions were collected.
Electrophoretic Analysis
Native gel electrophoresis was carried out with 7% PAGE 18. SDS-PAGE was performed with 12% polyacrylamide gel 19. The subunit molecular weight of the purified XO was determined by SDS-PAGE 20. Electrofocusing was performed and the isoelectric point (pI) value was calculated from a calibration curve 21, 22. Coomassie brilliant blue R-250 was used to stain the proteins.
Protein Determination
Protein content was determined by the dye (Coomassie Brilliant Blue G-250) binding assay method using BSA as a standard protein 23.
Results and Discussion
Purification of Sheep Liver Xanthine Oxidase
Xanthine oxidase was a commercially important enzyme with wide area of applications 24, 25. Sheep liver XO was purified using a purification scheme consisting of the following steps, mixing crude extract with n-butanol, acetone precipitation, anion-exchange chromatography on DEAE-cellulose column and gel filtration chromatography on Sephacryl S-300 column. The method is relatively short and involves only two chromatographic steps that seem to be simple and convenient method. Different purification methods of XO were reported as that of rat liver XO 26, 27 and buffalo milk XO 28. The elution profile of DEAE-cellulose column (Figure 1a) indicated one peak containing XO activity that XO was eluted as single peak at 0.05M NaCl and designated sheep liver xanthine oxidase (SLXO) Figure 1b). After chromatography on the Sephacryl S-300 column, SLXO was purified 31.8-fold with a specific activity of 3.5 units / mg protein and 24.1% recovery (Table 1). Buffalo milk xanthine oxidase; 10.66 unit/mg 28, bovine milk xanthine oxidase; 1.086 unit/mg 24, 0.3 unit/mg 29 and 15.8 unit/mg 30, human liver xanthine oxidase; 0.00061 unit/mg 31, and 0.036 unit/mg 32, buffalo liver xanthine oxidase; 7.2 unit/mg 33, rat liver xanthine oxidase; 14 unit/mg 27 and Arthrobacter M3 was 8.6 unit/mg 13.
Table 1. A typical purification scheme of the sheep liver xanthine oxidase (SLXO):| Purification steps | Total protein (mg) | Total Activity (unit) | Specific Activity | Yield (%) | Fold Purification |
| n-Butanol extract | 145.8 | 17.4 | 0.11 | 100 | 1.0 |
| Acetone fraction | 105 | 14 | 0.13 | 80.4 | 1.2 |
| DEAE-cellulose fraction | 15 | 8.3 | 0.55 | 47.7 | 5.0 |
| Sephacryl S-300 fraction | 1.2 | 4.2 | 3.5 | 24.1 | 31.8 |
Figure 1.A typical elution profile for the chromatography of the sheep liver acetone fraction on DEAE-cellulose column (6 cm x 2.4 cm i.d.) previously equilibrated with 0.02 M Tris-HCI buffer, pH 7.6 containing 0.1 mM EDTA. The proteins were eluted by a stepwise gradient of NaCl ranging from 0 to 1 M in the equilibration buffer and 5 ml fractions were collected at a flow rate of 60 ml / h. (b) A typical elution profile for the chromatography of the sheep liver DEAE-cellulose fraction on Sephacryl S-300 column (142 cm x 2.4 cm i.d.) previously equilibrated with 0.02 M Tris-HCI buffer, pH 7.6 containing 0.1 mM EDTA. The proteins were eluted by the same buffer and 2 ml fractions were collected at a flow rate of 30 ml / h.
Molecular Weight Determination and Electrophoretic Analysis of SLOX
Electrophoretic analysis of n-butanol extract, DEAE-cellulose fraction and Sephacryl S-300 purified fraction of SLXO on 7 % native PAGE revealed single protein band corresponded the enzyme activity band of the purified xanthine oxidase enzyme (Figure 2a). Gel filtration chromatography depicted only one activity peak, with a native molecular mass of 150 kDa. The subunit molecular weight of SLXO was determined by SDS-PAGE (Figure 2b) to be 75 kDa which revealed that the SLXO consists of two homodimer subunits. Many XO were reported to have dimeric structure such as 300 kDa for mouse liver 34, human liver 32 and rat liver 26. The xanthine oxidase from the bacterium Arthrobacter M3 showed two polypeptides with SDS-PAGE of molecular weights of 35 kDa and 100 kDa 13. The isoelectric point of SLXO enzyme was estimated by isoelectric focusing PAGE (Figure 2c) as two major molecular species with a value of 5.6 and 5.8. Isoelectric pH of XO in buffalo liver were 6 and 6.2 33, mouse liver was 6.7 34 and rat liver were 6.13, 6.23 and 6.07 27.
Figure 2.(a) Protein and XO isoenzyme pattern of sheep liver xanthine oxidase (SLXO) on 7 % native PAGE: (1) n-butanol extract, (2) DEAE-cellulose fraction, (3) Sephacryl S-300 purified fraction, and (4) SLXO isoenzyme pattern. (b) Subunit molecular weight determination by electrophoretic analysis of SLXO on 12 % SDS-PAGE: (1) Molecular weight marker proteins and (2) Purified SLXO. (c) Isoelectrofocusing: (1) isoelectric point (pI) marker proteins and (2) The purified sheep liver xanthine oxidase SLXO.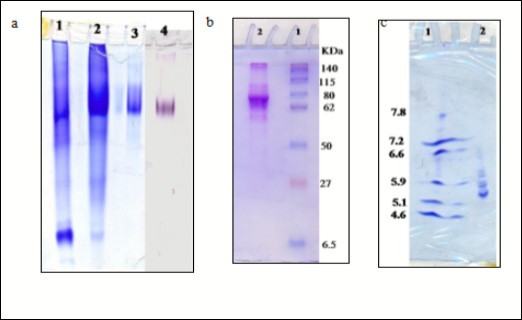
Determination of SLXO Optimum pH and Km Value
The effect of pH on the activity of sheep liver xanthine oxidase SLXO was examined in 0.05 M potassium phosphate buffer, pH (5.8-7.0) and 0.05 M Tris–HCl buffer, pH (7.2-9.0). The pH profile of SLXO displayed an optimum activity at pH 7.6 (Figure 3a). The optimum pH of the rabbit liver XO was found at pH 8.1 34. The Km value was calculated from the Lineweaver-Burk plot for the reciprocal of the reaction velocity (1/v) and substrate concentration (1/(S)) (Figure 3b) using xanthine as substrate. SLXO enzyme has a Km value of 0.9 mM (Figure 3b) indicating the high affinity of SLXO toward xanthine. Various Km values for XO were reported as; 1.1 mM xanthine for buffalo liver 33, 3.4 µM xanthine for Mouse liver 34, 22 µM for rabbit liver 35 and 53 µM for rat liver XO was 27.
Figure 3.(a) Effect of pH on the purified sheep liver xanthine oxidase SLXO using 0.05 M potassium phosphate buffer, pH (5.8-7.0) and 0.05 M Tris–HCl buffer, pH (7.2-9.0). (b) Lineweaver-Burk plot relating the reciprocal of the reaction velocity of the purified SLXO to xanthine concentration in mM.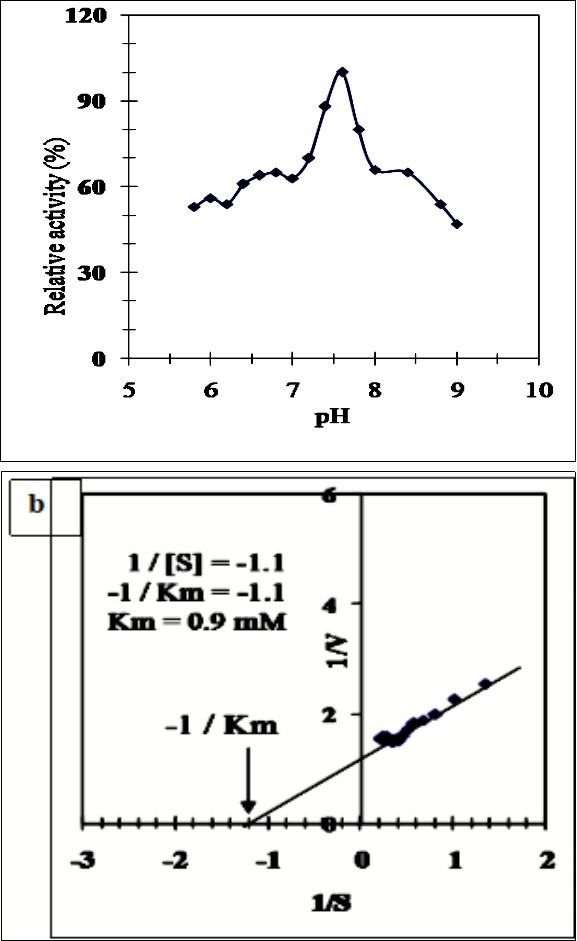
Effect of Divalent Cations and Various Inhibitors on SLXO
The effect of metal compounds on the purified SLXO activity was examined (Table 2). Divalent cations, such as CuCl2, MgCl2, MnCl2, CaCl2, CoCl2 and ZnCl2 inhibited the activity of sheep liver xanthine oxidase (SLXO) whereas FeCl2 and NiCl2 increased its activity. These results were consistent with that of cow milk xanthine oxidase which was inhibited by Cu+2, Hg+2 and Ag+ ions 36.
Table 2. Effect of divalent cations on the sheep liver xanthine oxidase (SLXO):| Reagent | Final Concentration (mM) | SLXO Residual activity (%) |
| Control | ----- | 100.0 |
| CoCl2 | 1.0 | 96.6 |
| 2.0 | 75.5 | |
| MnCl2 | 1.0 | 82.2 |
| 2.0 | 66.6 | |
| FeCl2 | 1.0 | 111.0 |
| 2.0 | 127.0 | |
| ZnCl2 | 1.0 | 68.8 |
| 2.0 | 44.4 | |
| CuCl2 | 1.0 | 75.0 |
| 2.0 | 46.6 | |
| NiCl2 | 1.0 | 108.3 |
| 2.0 | 113.5 | |
| MgCl2 | 1.0 | 102.8 |
| 2.0 | 88.2 | |
| CaCl2 | 1.0 | 91.4 |
| 2.0 | 73.6 |
Furthermore, the inhibition of purified SLXO activity by several inhibitors was studied (Table 3). Pre-incubation of the inhibitors for 5 min at 37˚C were carried out and the inhibition % was concluded as a proportion of a non-inhibited control. The purified SLXO was not inhibited with the serine protease inhibitor PMSF indicating that the active site of this isoenzyme doesn't contain a serine residue. No inhibition of SLXO by B mercaptoethanol and dithiothretol was observed indicating that no role of -SH groups in the enzyme activity. Iodoacetamide inhibited the purified SLXO activity which indicates that methionine, cysteine and histidine residues play important role in the structure and activity of the enzyme. The inhibition of the purified SLXO isoenzyme by the metal chelator EDTA indicates that SLXO isoenzyme is metalloenzyme. The inhibition of SLXO activity with K2Cr2O7 was probably due to strong oxidizing power of K2Cr2O7 that may cause oxidation of metal prosthetic groups in enzyme that important to enzyme activity. Allopurinol was found to be the most potent inhibitor of the purified SLXO. The effect of allopurinol concentrations on the purified SLXO indicated that 50% inhibition (I50) is caused by 0.1 mM allopurinol and the maximum inhibition of the enzyme (95.8%) was achieved by 1 mM allopurinol. However, full exploitation of these data required knowledge of the number of inhibitor molecules bound per enzyme molecule. Therefore, from the titration curve data (Figure 4a), a linear relationship was observed by constructing the Hill plot for the inhibition of the purified SLXO by allopurinol (Figure 4b). The slope of the Hill plot was found to be 1.19 indicating the existence of one binding site for allopurinol on the purified SLXO. The type of inhibition of the purified SLXO by allopurinol was found to be competitive type, whereas the presence of allopurinol did not alter the Vmax value but increased the Km value (Figure 4c). For the determination of the Ki value, the slopes of the reciprocal plots lines were plotted against the allopurinol concentration. The Ki value of the SLXO inhibition by allopurinol was found to be 0.06 mM directly from the intercept of the X axis of the plot (Figure 4d) indicating the potency of the allopurinol as inhibitor of XO. It is well known that, allopurinol is used as a drug for treating the elevated levels of uric acid in human by inhibiting xanthine oxidase and preventing the conversion of hypoxanthine and xanthine into uric acid.
Table 3. Effect of inhibitors on the purified sheep liver xanthine oxidase (SLXO):| Reagent | Final Concentration (mM) | SLXO Inhibition (%) |
| Control | ----- | 0.0 |
| Allopurinol | 1 mM | 95.8 |
| 2 mM | 100.0 | |
| Ethylenediamine tetraacetic acid (EDTA) | 2 mM | 18.0 |
| 5 mM | 25.8 | |
| DL-Dithiothreitol (DTT) | 2 mM | 1.4 |
| 5 mM | 3.5 | |
| Iodoacetamide | 2 mM | 17.5 |
| 5 mM | 31.9 | |
| Hydrogen peroxide (H2O2) | 2 mM | 12.5 |
| 5 mM | 22.5 | |
| β-Mercaptoethanol | 2 mM | 0.0 |
| 5 mM | 0.0 | |
| 1,10 Phenanthroline | 2 mM | 1.8 |
| 5 mM | 4.7 | |
| Phenylmethylsulfonyl-fluoride (PMSF) | 2 mM | 0.0 |
| 5 mM | 0.0 | |
| Potassium cyanide (KCN) | 2 mM | 0.0 |
| 5 mM | 0.0 | |
| Potassium dichromate (K2Cr2O7) | 2 mM | 57.4 |
| 5 mM | 88.7 | |
| Sodium azide (NaN3) | 2 mM | 10.0 |
| 5 mM | 27.2 | |
| Sodium dodecyl sulfate (SDS) | 2 mM | 14.5 |
| 5 mM | 26.3 |
Figure 4.(a) Titration curve for inhibition of the purified SLXO by varying concentrations of allopurinol. (b) Hill plot for inhibition of the purified SLXO by varying concentrations of allopurinol. (c) Lineweaver-Burk plots showing the type of inhibition of the purified SLXO by allopurinol. (d) Determination of the inhibition constant (Ki) value for the inhibition of the purified SLXO by allopurinol.
Conclusion
In conclusions, the present study is the first study to report purification of sheep liver xanthine oxidase (SLXO). This study presents a simple, convenient and reproducible method for the purification of well characterized xanthine oxidase from sheep liver as locally available sources. Production of this enzyme on large scale will make it suitable for various medical applications as preparation of SOD diagnostic kit and a detection reagent for nucleotidase, purines, adenosine deaminase and phosphates.
Acknowledgements
The authors would gratefully acknowledge financial support provided by the National Research Centre, Egypt.
References
- 1.Borges F, Fernandes E, Roleira F. (2002) Progress towards the discovery of xanthine oxidase inhibitors., Current medicinal chemistry 9(2). 195-217.
- 2.Stein B W, Kirk M L. (2015) Electronic structure contributions to reactivity in xanthine oxidase family enzymes. , J Biol Inorg Chem 20, 183-194.
- 3.MRA Correia, Otrelo-Cardoso A R, Schwuchow V, Sigfridsson Clauss KG, Haumann M et al. (2016) The Escherichia coli periplasmic aldehyde oxidoreductase is an exceptional member of the xanthine oxidase family of molybdoenzymes. , ACS Chem Biol 11, 2923-2935.
- 4.Zhang C, Zhang G, Liao Y, Gong D. (2017) Myricetin inhibits the generation of superoxide anion by reduced form of xanthine oxidase. , Food Chem 221, 1569-1577.
- 5.Zhang C, Zhang G, Pan J, Gong D. (2016) Galangin competitively inhibits xanthine oxidase by a ping-pong mechanism. , Food Res Int 89, 152-160.
- 6.Vorbach C, Roger H, Capecchi M R. (2003) Xanthine oxidoreductase is central to the evolution and function of the innate immune system. , Trends Immunol 24(9), 512-517.
- 7.Marcus S R, Dharmalingam M. (2014) Iron, Oxidative Stress and Diabetes. In V. Preedy, (eds.). Diabetes, Elsevier Inc. PP 51-64.
- 8.Topham R W, Walker M C, Calisch M P. (1982) Liver xanthine dehydrogenase and iron mobilization. , Biochem Biophys Res Com 109(4), 1240-1246.
- 9.Parks D, Granger D. (1986) Xanthine oxidase: biochemistry, distribution and physiology. Acta physiologica Scandinavica Supplementum 548 87.
- 10.Battelli M G, Bolognesi A, Polito L. (2014) Xanthine oxidoreductase in drug metabolism: Beyond a role as a detoxifying enzyme. , Biochim Biophys Acta Mol Basis Dis 1842, 1502-1517.
- 11.Haddadian Z, Eyres G T, Carne A, Everett D W, Bremer P. (2017) Impact of different milk fat globule membrane preparations on protein composition, xanthine oxidase activity, and redox potential. , Int Dairy J 64, 14-21.
- 12.Jarasch E, Bruder G, Heid H. (1985) Significance of xanthine oxidase in capillary endothelial cells. , Acta physiologica Scandinavica Supplementum 548, 39-46.
- 13.Zhang Y, Xin Y, Yang H, Zhang L, Xia X et al. (2012) Novel affinity purification of xanthine oxidase from Arthrobacter M3. , J Chromat B 906, 19-24.
- 14.Pacher P, Nivorozhkin A, Szabó C. (2006) herapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. , Pharmacological reviews 58(1), 87-114.
- 15.Agarwal A, Banerjee U. (2009) Screening of xanthine oxidase producing microorganisms using nitroblue tetrazolium based colorimetric assay method. , Open Biotech J 3, 46-49.
- 16.Özer N, Muftüoglu M, Ögus I H. (1998) A simple and sensitive method for the activity staining of xanthine oxidase. , J. Biochem Biophys Meth 36(2), 95-100.
- 17.Oida S, Sone M, Sasaki S. (1984) Purification of swine kidney alkaline phosphatase by immunoaffinity chromatography. , Anal Biochem 140(1), 117-120.
- 18.Smith I. (1969) Acrylamide gel disc electrophoresis. In "Electrophoretic techniques" (Edited by Smith, I.), Academic press. , New York 365-515.
- 19.Laemmli U K. (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. , Nature 227, 680-685.
- 20.Weber K, Osborn M. (1969) The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. , J Biol Chem 244(16), 4406-4412.
- 21.O'Farrell P H. (1975) High resolution two-dimensional electrophoresis of proteins. , J Biol Chem 250(10), 4007-4021.
- 22.Ubuka T, Masuoka N, Yoshida S, Ishino K. (1987) Determination of isoelectric point value of 3-mercaptopyruvate sulfurtransferase by isoelectric focusing using ribonuclease A-glutathione mixed disulfides as standards. , Anal Biochem 167(2), 284-289.
- 23.Bradford M M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. , Anal Biochem 72, 248-254.
- 24.Özer N, Müftüoglu M, Ataman D, Ercan A, Ögüs I H. (1999) Simple, high-yield purification of xanthine oxidase from bovine milk. , J Biochem Biophys Meth 39(3), 153-159.
- 25.Antunes M F, Eggimann F K, Kittelmann M, Lütz S, Hanlon S P et al. (2016) Human xanthine oxidase recombinant in E. coli: A whole cell catalyst for preparative drug metabolite synthesis. , J Biotechnol 235, 3-10.
- 26.Maia L, Mira L. (2002) Xanthine oxidase and aldehyde oxidase: A simple procedure for the simultaneous purification from rat liver. , Arch Biochem Biophys 400(1), 48-53.
- 27.McManaman J, Shellman V, Wright R, Repine J. (1996) Purification of rat liver xanthine oxidase and xanthine dehydrogenase by affinity chromatography on benzamidine-sepharose. , Arch Biochem Biophys 332(1), 135-141.
- 28.HMM Masoud, Darwish D A, Abdel-Monsef M M, Helmy M S, Ibrahim M A. (2017) Xanthine oxidase from milk of the water buffalo (Bubalus bubalis): Purification, characterization and application in SOD assay diagnostic kit. , Res J Pharmac Biol Chem Sci 8(3), 1735-1744.
- 29.Vella M, Hunter T, Farrugia C, Pearson A R, Hunter G. (2014) Purification and characterisation of xanthine oxidoreductases from local bovids in Malta. , Adv Enz Res 2, 54-63.
- 30.Beyaztaş S, Arslan O. (2015) Purification of xanthine oxidase from bovine milk by affinity chromatography with a novel gel. , J Enz Inhib Medic Chem 30(3), 442-447.
- 31.Moriwaki Y, Yamamoto T, Suda M, Nasako Y, Takahashi S et al. (1993) Purification and immunohistochemical tissue localization of human xanthine oxidase. , Biochim Biophys Act Prot Struct Mol Enzymol 1164(3), 327-330.
- 32.Krenitsky T A, Spector T, Hall W W. (1986) Xanthine oxidase from human liver: purification and characterization. , Arch Biochem Biophy 247(1), 108-119.
- 33.Ibrahim M A, HMM Masoud, Darwish D A, Esa S S, SAM Zaahkouk. (2015) Purification and characterization of xanthine oxidase from liver of the water buffaloBubalusbubalis. , J Appl Pharmac Sci 5(11), 63-68.
- 34.Carpani G, Racchi M, Ghezzi P, Terao M, Garattini E. (1990) Purification and characterization of mouse liver xanthine oxidase. , Arch Biochem Biophys 279(2), 237-241.
Cited by (1)
- 1.Satyam Satyam, Patra Sanjukta, 2025, Xanthine oxidase driven bio-Fenton system for advanced pollutant degradation in sustainable wastewater treatment, International Journal of Biological Macromolecules, 313(), 144323, 10.1016/j.ijbiomac.2025.144323
