Improving the Efficiency of GF-120 Baits in Attracting BactroceraZonata by Adding Ammonium Compounds with Particular Emphasis on pH level
Abstract
The peach fruit fly, Bactrocerazonata (Saunders) is a serious pest attacking a wide range of fruits. Field experiments were carried out, at Mansoura district, Dakahlia Governorate, Egypt to evaluate the efficiency of di- ammonium phosphate, ammonium carbonate and ammonium acetate in enhancing GF-120, as insecticidal bait, for B. zonata based on their pH level under high and low population levels of B. zonata. Results showed that di-ammonium phosphate enhanced the attractiveness of GF-120 the most, followed by ammonium carbonate and ammonium acetate. Without adding any of the ammonium compounds to the GF-120 bait, the bait attracted the fewest B. zonata flies regardless of population levels. As the concentrations of ammonium compounds increased, the pH-level increased as well in the prepared GF-120 solutions, resulting in increased numbers of B. zonata flies captured. In contrast to males, females of B. zonata were more responsive to increase concentrations of the three ammonium compounds tested. Accordingly, all treatments attracted females more than males. The sex ratio (as number of attracted females per one male) was generally higher under low than high fly population levels.
Author Contributions
Academic Editor: Emily Fontenot, Insect Pest Control Laboratory, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, IAEA, Wagramerstrasse 5, A-1400 Vienna, Austria.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Nabil Mohamed Ghamin
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Phytophagous insects use a variety of sensory capabilities to orient and recognize the appropriate host plants 1. Tephritid fruit flies (Diptera: Tephritidae) may use chemical stimuli in the form of nutrients to orient towards host plants 2. Food sources that are rich in nitrogen have a strong influence on the physiology and behaviour of tephritid flies 3, 4. This behaviorally-based tactic targets female fruit flies primarily based on the female’s need of protein for ovarian development and egg production 5, 6.
Use of proteinaceous bait sprays (protein mixed with a toxicant) are one effective method for suppressing populations of many tephritid species 7, 8, 9. Bait sprays mainly work on female fruit flies, which are strongly attracted to protein source from which ammonia emanate, causing flies to ingest a lethal dose of insecticide together with the protein 10, 11. Response of fruit flies to traps baited with synthetic lures versus those with liquid protein bait tends to be variable and host/population level may be the cause in such variation 5, 6, 7, 8, 9, 10, 11, 12.
Baited insecticides are an attractive alternative to conventional pesticides that used in fruit fly control, in part because the environmental impact is reduced compared with broadcast foliar sprays. GF-120 GF has been the most frequently tested bait against fruit flies 9, 13, 14. It contains a much lower concentration of active ingredient than in broadcast spray formulations, such as in the unbaited SpinTor formulation of spinosad (0.2% compared with 22.8% in SpinTor) and it has a lower effect on non-target insects 7, 13, 15. GF-120 contains 1% ammonium acetate (wt:vol) as an attractant 16. Spinosad is an insecticide derived from fermentation products of the bacterium, Saccharopolyspora spinosa Mertz and Yao that has a high safety profile 17.
Bait sprays using GF-120 became the primary tool for covering a wide area and for suppressing the tephritid fruit fly populations 18, 19, 20. Although protein baits do not attract flies from long distances, GF-120 apparently is attractive to some species and has been used to suppress larval infestations in many fruit orchards 21, 22. In contrast, some researchers reported that GF-120 is not highly attractive to some fruit flies (i.e.Rhagoletis flies) 14, 18, 23. Accordingly, Pelz-Stelinski et al. 19 emphasized the need to improve the efficiency of GF-120 bait to be more attractive.
However, given the zero tolerance for fruit flies at fruit harvest, a higher level of control is required. Therefore, increasing attractiveness of GF-120 is required to increase its commercial use for attracting fruit flies. This increase would be expected to promote greater likelihood that flies ingest spinosad, thus improving the efficacy of this bait spray. On another hand, ammonia is associated with protein-rich foods and has long been known to attract fruit flies 24, 25, 26, 27. Yee and Landolt 28 found that increasing the concentration of ammonia in lures significantly increased their attraction to apple maggot fly, Rhagoletispomonella (Walsh). Indeed, adding more ammonium acetate to GF-120 enhanced its attractiveness to some eastern fruit fly, Rhagoletiscingulata (Loew) 19. On the other hand, pH-level of the baits plays a fundamental role in attracting fruit flies, since the effectiveness of bait is diminished as the pH-level decreased 29, 30, 31, 32, 33. This information therefore could be used in chemical analysis for identification of new attractants from preferred bait formulations.
In Egypt, the peach fruit fly, Bactrocerazonata (Saunders), is a serious pest attacking a wide range of fruits that differ in their ripening time stage during the year. It is a polyphagus insect attacking more than 50 species of fruit and vegetable crops as well as wild host plants 34. It causes 25-50% damage to fruits particularly in the summer season 35. This fly could be effectively controlled using GF-120 enhanced with ammonium compounds, but the attractiveness of these baits needs to be determined.
The purposes of this study were to 1) enhance the ability of GF-120 to attract B. zonata by adding di- ammonium phosphate, ammonium carbonate and ammonium acetate, and to 2) study the relationship between pH-level and captures of B. zonata flies on traps.
Materials and Methods
Materials and Treatments
The GF-120 (Conserve 0.024% CB) was obtained from Dow AgroSciences, England, whereas di-ammonium phosphate ((NH4)2 HPO4), ammonium carbonate ((NH4)2 CO3) and ammonium acetate (CH3COONH4) were received from El-Naser for Drugs and Chemicals Company. Each of the three ammonium compounds was added to a 5% GF-120 solution (vol/vol) at 0.5, 1.0, 2.0 and 3.0% (wt/vol). All treatments (GF-120 + compound) were compared with a GF-120 bait only control.
Field Trials
To evaluate the efficiency of di-ammonium phosphate, ammonium carbonate and ammonium acetate in enhancing the GF-120, as a bait for B. zonata, experiments were conducted in guava, Psidium guajava L. (as high B. zonata population level; whereas mean flies trapped per day values (FTDs) ranged between 1.75 and 10.33) and navel orange, Citrus sinensis L. (as low population level; whereas mean FTDs ranged between 0.44 and 2.40) orchards located in the experimental farm of Mansoura University, Dakahlia governorate, Egypt. The cultivated areas were about seven feddans for guava and eight feddans for navel orange (1 feddan = 4200 m2). Experiments were carried out from 9-20 October 2017 in guava orchard and from 20-31 October 2017 in navel orange orchard.
Following the approach of Yee 36 to determine the effects of adding ammonium compounds to GF-120 on attraction of R. pomonella in Washington State using sticky yellow panel traps, this study used the modified Nadel traps 37 that baited with the GF-120 mixed with varying concentrations of ammonium compounds in capturing B. zontata flies. Each treatment consisted of 250 milliliters installed in a trap and replicated four times. Traps were distributed inside each orchard (guava or navel orange) in a completely randomized design. Traps were hanged at a height of 1.5-2.0 meters above the ground in the shade under trees. The distance between every two adjacent traps was about 20 meters to avoid the interaction between lures.
Traps were inspected every two days over 12 days after hanging for flies. Captured female and male flies were counted and recorded as FTDs. The captured flies were removed from traps with no renewal of the bait solution. To reduce trap position effects (i.e., due to light, wind, heat, and other factors), traps were rotated at every inspection.
Estimating pH Levels
Fifty milliliters of each treatment were transferred to laboratory for estimating pH level. Samples were taken when preparing the treatments (as fresh bait) and at the end of experiment in guava and navel orange treatments. These samples were measured by Jenway 3510 pH meter.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) at probability level of 0.05. Regression analysis was also performed between concentration of ammonium compounds and pH in addition; regression analysis was performed between each of concentration and pH and FTDs. All analyses were performed using CoHort Software 38.
Results
By Adding Di-Ammonium Phosphate
Adding di-ammonium phosphate at the concentration of 2.0% to GF-120 resulted in the highest captures of B. zonata flies under high population (mean FTD was 10.33±0.59; F = 77.78 and P <0.01). Under low population, adding this compound at the concentration of 1.0% resulted in the highest captures (FTD = 1.67±0.40; F = 10.80 and P <0.01). In contrast, the GF-120 alone control caught the fewest B. zonata flies under high (FTD = 1.75±0.38) or low (0.44±0.14) populations. The attraction to traps baited with 1.0, 2.0 and 3.0% di-ammonium phosphate decreased two days after trap deployment (Table 1).
Table 1. Mean number (±SD) of attracted B. zonata adults to GF-120 enhanced by di-ammonium phosphate at different concentrations under two levels (high and low) of pest population in relation to pH-level.| Conc.% | FTD after (in days) | pH | |||||||
| 2 | 4 | 6 | 8 | 10 | 12 | Mean | Fresh bait | At the end | |
| Under high population level | |||||||||
| 0.0 | 0.75±0.50c | 0.88±0.48d | 2.13±0.48c | 2.25±0.29c | 1.75±0.95b | 2.75±0.29b | 1.75±0.38c | 4.51 | 3.92 |
| 0.5 | 1.63±0.48c | 3.50±0.41c | 3.75±0.65b | 3.63±1.44b | 2.75±1.55b | 1.13±0.63c | 2.73±0.79c | 5.89 | 4.58 |
| 1.0 | 16.88±2.29b | 9.25±1.19ab | 4.25±1.19b | 3.75±0.87b | 1.50±1.08b | 0.75±0.64c | 6.06±0.66b | 7.04 | 4.98 |
| 2.0 | 22.75±2.66a | 9.75±1.32a | 5.88±0.75a | 7.38±0.25a | 7.25±0.50a | 9.00±1.22a | 10.33±0.59a | 7.18 | 6.12 |
| 3.0 | 23.25±3.28a | 7.88±1.31b | 2.38±0.63c | 2.00±1.08c | 1.63±0.75b | 2.63±1.25b | 6.63±1.20b | 7.50 | 5.94 |
| Under low population level | |||||||||
| 0.0 | 0.13±0.25c | 0.00±0.00d | 1.63±0.48c | 0.63±0.48a | 0.00±0.00a | 0.25±0.29b | 0.44±0.14c | 4.51 | 3.69 |
| 0.5 | 0.38±0.48c | 0.38±0.25d | 3.00±0.41b | 0.25±0.50a | 0.50±0.41a | 0.88±0.48a | 0.90±0.25b | 5.89 | 4.02 |
| 1.0 | 1.75±0.71a | 2.13±0.85a | 4.38±0.63a | 0.88±0.85a | 0.63±0.48a | 0.25±0.29b | 1.67±0.40a | 7.04 | 4.60 |
| 2.0 | 1.13±0.25ab | 1.38±0.63bc | 1.63±0.25c | 0.75±0.64a | 0.50±0.68a | 0.00±0.00b | 0.90±0.22b | 7.18 | 5.22 |
| 3.0 | 0.75±0.50bc | 1.13±0.25c | 1.25±0.50c | 0.63±0.48a | 0.75±0.96a | 0.25±0.29b | 0.79±0.28bc | 7.50 | 6.32 |
The highest attraction of B. zonata flies was recorded when pH level of the fresh bait was relatively high (more than 7.00). On the other hand, pH-levels were lower at the low than high population experiment (Table 1).
There was a positive relationship between concentration of di-ammonium phosphate and the resulted pH level in the prepared solution. By the end of experiment, each increase in the concentration of di-ammonium phosphate by one percent increased pH by 0.88, 0.71 and 0.86 degrees in fresh bait, under high population level and low population level, respectively (Figure 1).
Figure 1.Relationship between concentrations of di-ammonium phosphate added to GF-120 and pH levels in fresh bait or at the end of experiment (under high and low levels of B. zonata population).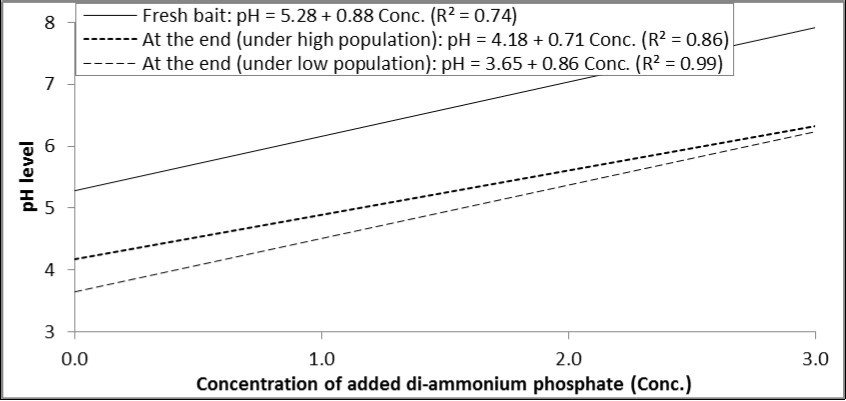
The attracted B. zonata flies (females, males or total numbers) were more affected by pH levels in comparison with concentration of di-ammonium phosphate. The determination coefficient values (R2) were generally higher in pH compared with those in concentrations of di-ammonium phosphate (Table 2).
Table 2. Relationships between attracted B. zonata flies (as FTD) and each of pH level (in fresh bait and at the end of experiment) and concentrations of di-ammonium phosphate added to GF-120 under two levels (high and low) of pest population.| Factor | Sex | At high population level | At low population level | |||
| Relationship | R2 | Relationship | R2 | |||
| pH level | Fresh bait | Females | FTD = -6.47 + 1.58 pH | 0.70 | FTD = -0.36 + 0.16 pH | 0.27 |
| Males | FTD = -2.73 + 0.71 pH | 0.60 | FTD = -0.02 + 0.05 pH | 0.22 | ||
| Total | FTD = -9.21 + 2.29 pH | 0.68 | FTD = -0.37 + 0.20 pH | 0.31 | ||
| At the end of experiment | Females | FTD = -8.48 + 2.37 pH | 0.89 | FTD = 0.43 + 0.05 pH | 0.02 | |
| Males | FTD = -3.43 + 1.03 pH | 0.72 | FTD = 0.28 - 0.002 pH | 0.0004 | ||
| Total | FTD = -11.92 + 3.41 pH | 0.85 | FTD = 0.73 + 0.04 pH | 0.01 | ||
| Concentration | Females | FTD = 1.65 + 1.54 pH | 0.64 | FTD = 0.62 + 0.03 pH | 0.01 | |
| Males | FTD = 1.11 + 0.57 pH | 0.36 | FTD = 0.27 + 0.003 pH | 0.001 | ||
| Total | FTD = 2.76 + 2.11 Conc. | 0.55 | FTD = 0.90 + 0.03 Conc. | 0.01 | ||
On the other hand, B. zonata females were more responsive to the increase of pH level and concentration percentages of di-ammonium phosphate than males. Under high population level, each increase of pH level in fresh baits by one degree increased the attracted females and males (as FTDs) by 1.58 and 0.71. By the end of the experiment, each increase of pH level increased FTDs of females and males by 2.37 and 1.03. With respect to the concentration of di-ammonium phosphate, each increase by one percent increased the attracted females and males by 1.54 and 0.57 (Table 2).
Under low population level, each increase of pH level in fresh baits increased the attracted females and males by 0.16 and 0.05 FTDs; in the cases of pH at the end of experiment and concentration percentages, these relations were very weak (Table 2).
All of the tested treatments attracted more females than males of B. zonata. The highest number of females per one male was recorded when adding di-ammonium phosphate with the concentration of 3.0% under high population (2.62 females; F = 7.63 and P <0.01) and with the concentration of 1.0% under low population (3.74 females; F = 23.52 and P <0.01). In contrast, the lowest number of females per one male was recorded with the concentration of 0.5% under high (1.42 females) and low (1.37 females) populations. On the other hand, the number of attracted females per one male was generally high under low population in comparison with high population (Figure 2).
Figure 2.Sex ratio (as No. of females/ 1male) of attracted B. zonata flies to GF-120 enhanced by di-ammonium phosphate at different concentrations under two levels (high and low) of pest population (In each population level; means have the same letter did not differ significantly at the probability of 0.05).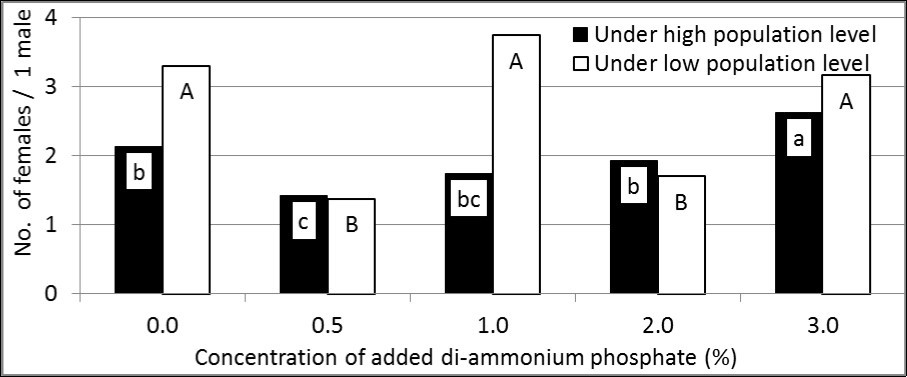
By Adding Ammonium Carbonate
Under high population level, adding ammonium carbonate with the concentration of 3.0% to GF-120 resulted in the highest number of B. zonata fly captured (mean FTD was 8.43±0.42; F = 45.06 and P <0.01); under low population, adding this compound at the concentration of 1.0% attracted the highest number of flies (FTD = 1.50±0.18; F = 11.84 and P <0.01). The GF-120 control attracted the fewest B. zonata flies under high and low population levels (Table 3).
Table 3. Mean number (±SD) of attracted B. zonata adults to GF-120 enhanced by ammonium carbonate at different concentrations under two levels (high and low) of pest population in relation to pH level.| Conc.% | FTD after (in days) | pH | |||||||
| 2 | 4 | 6 | 8 | 10 | 12 | Mean | Fresh bait | At the end | |
| Under high population level | |||||||||
| 0.0 | 0.75±0.50c | 0.88±0.48d | 2.13±0.48d | 2.25±0.29c | 1.75±0.95b | 2.75±0.29b | 1.75±0.38c | 4.51 | 3.92 |
| 0.5 | 23.50±2.65a | 9.63±0.95a | 4.00±0.71cd | 2.13±1.55c | 0.25±0.50c | 0.38±0.25c | 6.65±0.80ab | 6.90 | 4.63 |
| 1.0 | 4.75±0.50b | 5.38±1.31b | 4.88±1.18bc | 2.75±0.50c | 1.38±0.85bc | 0.75±0.29c | 3.31±0.68bc | 7.45 | 4.96 |
| 2.0 | 5.50±0.71b | 5.38±1.31b | 9.00±3.48a | 6.25±1.76b | 1.63±0.63b | 1.38±0.48c | 4.85±1.29b | 7.80 | 5.13 |
| 3.0 | 5.38±1.11b | 3.38±0.48c | 6.88±0.75ab | 8.95±0.71a | 9.63±0.95a | 16.38±1.75a | 8.43±0.42a | 8.01 | 6.62 |
| Under low population level | |||||||||
| 0.0 | 0.13±0.25ab | 0.00±0.00c | 1.63±0.48bc | 0.63±0.48cd | 0.00±0.00c | 0.25±0.29a | 0.44±0.14b | 4.51 | 3.69 |
| 0.5 | 0.63±0.48a | 0.75±0.29a | 1.38±1.11c | 1.25±0.65bc | 0.25±0.29bc | 0.13±0.25a | 0.73±0.40b | 6.90 | 4.48 |
| 1.0 | 0.50±0.58ab | 0.38±0.25b | 7.50±0.91a | 0.13±0.25d | 0.25±0.50bc | 0.25±0.29a | 1.50±0.18a | 7.45 | 6.83 |
| 2.0 | 0.00±0.00b | 0.00±0.00c | 2.50±0.58b | 1.63±0.25ab | 3.00±0.71a | 0.13±0.25a | 1.21±0.26a | 7.80 | 7.21 |
| 3.0 | 0.00±0.00b | 0.00±0.00c | 0.25±0.29d | 2.13±0.95a | 0.75±0.50b | 0.00±0.00a | 0.52±0.27b | 8.01 | 5.72 |
The highest number of attracted B. zonata flies was recorded at 8.01 and 7.45 pH of the fresh bait. Also, the highest number of attracted flies was recorded at high pH levels at the end of experiment under high and low population levels; however, pH levels were 6.62 and 6.83 (Table 3).
Each increase in the concentration of ammonium carbonate by one percent increased pH by 0.94 degrees in the fresh solution, and increased it by 0.79 and 0.75 in the baits at the end of experiment under high and low population levels (Figure 3).
Figure 3.Relationship between concentrations of ammonium carbonate added to GF-120 and pH levels in fresh bait or at the end of experiment (under high and low levels of B. zonata population).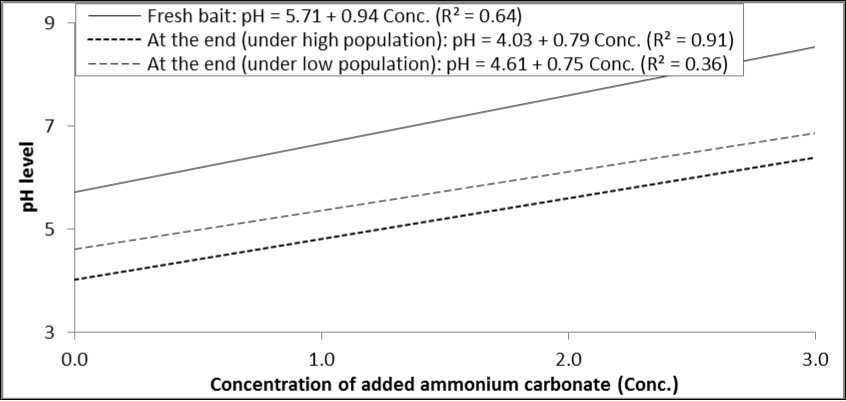
As in di-ammonium phosphate, the attracted B. zonata flies (females, males or total numbers) were more affected by pH level in comparison with concentration of ammonium carbonate; the determination coefficient values (R2) were generally higher in pH-degrees compared with those in ammonium concentrations (Table 4).
Table 4. Relationships between attracted B. zonata flies (as FTD) and each of pH level (in fresh bait and at the end of experiment) and concentrations of ammonium carbonate added to GF-120 under two levels (high and low) of pest population.| Factor | Sex | At high population level | At low population level | |||
| Relationship | R2 | Relationship | R2 | |||
| pH level | Fresh bait | Females | FTD = -2.56 + 0.83 pH | 0.47 | FTD = -0.21 + 0.12 pH | 0.20 |
| Males | FTD = -1.50 + 0.47 pH | 0.53 | FTD = -0.05 + 0.04 pH | 0.18 | ||
| Total | FTD = -4.05 + 1.31 pH | 0.49 | FTD = -0.23 + 0.16 pH | 0.25 | ||
| At the end of experiment | Females | FTD = -4.03 + 1.43 pH | 0.67 | FTD = -0.59 + 0.22 pH | 0.73 | |
| Males | FTD = -1.88 + 0.73 pH | 0.61 | FTD = 0.05 + 0.03 pH | 0.13 | ||
| Total | FTD = -5.93 + 2.16 pH | 0.66 | FTD = -0.52 + 0.25 pH | 0.67 | ||
| Concentration | Females | FTD = 1.84 + 1.06 pH | 0.54 | FTD = 0.59 + 0.03 pH | 0.01 | |
| Males | FTD = 1.12 + 0.51 pH | 0.46 | FTD = 0.26 – 0.01 pH | 0.006 | ||
| Total | FTD = 2.95 + 1.58 Conc. | 0.52 | FTD = 0.85 + 0.02 Conc. | 0.003 | ||
Also, B. zonata females were more responsive to the increase of pH level and concentration percentage of ammonium carbonate in comparison with males (Table 4). The b-values in the mathematical relationship between pH levels and concentration percentages of ammonium carbonate and FTD were higher in the case of females in comparison with males in all cases.
The highest number of attracted females per one male was recorded with GF-120 alone under high population (2.13 females; F = 1.19 and P = 0.357) and when adding ammonium carbonate to the insecticidal bait with the concentration of 2.0% under low population (7.07 females; F = 71.45 and P <0.01). In contrast, the lowest number of attracted females per one male was recorded with the concentration of 1.0% under high population (1.49 females with no significant differences between concentrations) and with the concentration of 0.5% under low population (1.35 females). The number of attracted females per one male was generally higher under low population levels than under high population levels (Figure 4).
Figure 4.Sex ratio (as No. of females/ 1male) of attracted B. zonata flies to GF-120 enhanced by ammonium carbonate at different concentrations under two levels (high and low) of pest population (In each population level; means have the same letter did not differ significantly at the probability of 0.05).
By Adding Ammonium Acetate
Ammonium acetate at 0.5 and 3.0% in GF-120 were the most effective treatments for attracting B. zonata flies under high population level (mean FTDs were 4.33±0.44 and 4.23±0.87; F = 26.17 and P <0.01). Under low population, adding this compound at 3.0% attracted the highest number of flies (FTD = 2.40±0.24; F = 72.95 and P <0.01). In contrast, GF-120 alone and GF-120 with 1.0% ammonium acetate attracted the fewest B. zonata flies under high (1.75±0.38 and 1.65±0.31) or low (0.44±0.14 and 0.50±0.18) population levels (Table 5).
Table 5. Mean number (±SD) of attracted B. zonata adults to GF-120 enhanced by ammonium acetate at different concentrations under two levels (high and low) of pest population in relation to pH-degree.| Conc.(%) | FTD after (in days) | pH | |||||||
| 2 | 4 | 6 | 8 | 10 | 12 | Mean | Fresh bait | At the end | |
| Under high population level | |||||||||
| 0.0 | 0.75±0.50b | 0.88±0.48c | 2.13±0.48c | 2.25±0.29c | 1.75±0.95bc | 2.75±0.29c | 1.75±0.38c | 4.51 | 3.92 |
| 0.5 | 1.38±0.63b | 8.13±2.93a | 7.50±1.29a | 5.38±1.31a | 2.75±1.19b | 0.88±0.48d | 4.33±0.44a | 5.18 | 4.47 |
| 1.0 | 0.88±0.25b | 2.38±0.63bc | 3.38±1.18bc | 2.13±0.48c | 0.75±0.29c | 0.38±0.25d | 1.65±0.31c | 5.48 | 4.67 |
| 2.0 | 3.13±0.85a | 3.00±0.58bc | 3.50±0.41bc | 3.50±1.08b | 3.13±1.03b | 4.25±0.50a | 3.42±0.42b | 5.77 | 4.76 |
| 3.0 | 4.13±1.31a | 4.00±1.08b | 3.75±1.04b | 5.13±0.63a | 4.88±1.25a | 3.50±0.71b | 4.23±0.87a | 6.02 | 5.23 |
| Under low population level | |||||||||
| 0.0 | 0.13±0.25 | 0.00±0.00 | 1.63±0.48 | 0.63±0.48 | 0.00±0.00 | 0.25±0.29 | 0.44±0.14 | 4.51 | 3.69 |
| 0.5 | 0.75±0.29 | 0.50±0.41 | 1.25±0.50 | 1.00±0.41 | 0.38±0.25 | 0.25±0.29 | 0.69±0.08 | 5.18 | 4.33 |
| 1.0 | 0.00±0.00 | 0.00±0.00 | 2.13±0.48 | 0.50±0.41 | 0.13±0.25 | 0.25±0.29 | 0.50±0.18 | 5.48 | 4.53 |
| 2.0 | 0.25±0.29 | 0.25±0.29 | 4.38±0.75 | 0.38±0.48 | 0.25±0.29 | 0.75±0.29 | 1.04±0.25 | 5.77 | 4.82 |
| 3.0 | 0.13±0.25 | 0.00±0.00 | 11.00±1.08 | 1.75±0.29 | 0.63±0.63 | 0.88±0.48 | 2.40±0.24 | 6.02 | 5.16 |
On the other hand, the highest numbers of attracted B. zonata flies were coincided with the high pH level of the fresh bait (pH = 6.02) and at the end of experiment under high and low population levels (pH = 5.23 and 5.16). Generally, pH levels decreased under low population more than that under high population (Table 5).
As with the other two ammonium compounds, there was a positive relationship between concentration of ammonium acetate and the resulted pH level in the prepared solution. Statistically, each increase in the concentration of ammonium acetate by one percent increased pH by 0.45, 0.37 and 0.44 degrees in fresh bait and at the end of experiments under high and low population levels, respectively (Figure 5).
Figure 5.Relationship between concentrations of ammonium acetate added to GF-120 and pH levels in fresh bait or at the end of experiment (under high and low levels of B. zonata population).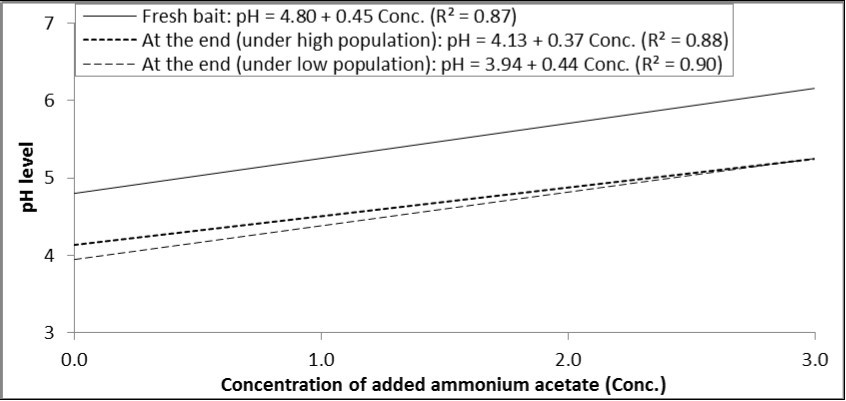
B. zonata flies under high population level were generally more affected by pH level in comparison with concentration of ammonium acetate (Table 6); the determination coefficient values (R2) were generally higher in pH levels compared with those in concentrations of di-ammonium phosphate. In contrast, the attracted B. zonata flies under low population level were generally more affected by concentration of ammonium acetate in comparison with pH levels.
Table 6. Relationships between attracted B. zonata flies (as FTD) and each of pH level (in fresh bait and at the end of experiment) and concentration of ammonium acetate added to GF-120 under two levels (high and low) of pest population.| Factor | Sex | At high population level | At low population level | |||
| Relationship | R2 | Relationship | R2 | |||
| pH level | Fresh bait | Females | FTD = -2.166 + 0.78 pH | 0.28 | FTD = -3.19 + 0.72 pH | 0.44 |
| Males | FTD = -1.43 + 0.45 pH | 0.30 | FTD = -1.38 + 0.32 pH | 0.87 | ||
| Total | FTD = -3.36 + 1.19 pH | 0.29 | FTD = -4.54 + 1.03 pH | 0.55 | ||
| At the end of experiment | Females | FTD = -2.79 + 1.05 pH | 0.34 | FTD = -3.05 + 0.83 pH | 0.52 | |
| Males | FTD = -1.58 + 0.56 pH | 0.31 | FTD = -1.21 + 0.34 pH | 0.90 | ||
| Total | FTD = -4.15 + 1.57 pH | 0.33 | FTD = -4.24 + 1.17 pH | 0.64 | ||
| Concentration | Females | FTD = 1.60 + 0.36 Conc. | 0.25 | FTD = 0.11 + 0.45 Conc. | 0.71 | |
| Males | FTD = 0.70 + 0.23 Conc. | 0.34 | FTD = 0.10 + 0.16 Conc. | 0.98 | ||
| Total | FTD = 2.32 + 0.58 Conc. | 0.28 | FTD = 0.22 + 0.61 Conc. | 0.82 | ||
On the other hand, B. zonata females were more responsive than males to the increase of pH levels and concentration percentages of ammonium acetate. This is explained by the b-values in the mathematical relationship between each of pH levels and concentration percentages of ammonium acetate and FTDs; which were higher in the case of females than males in all cases (Table 6).
The highest number of attracted females per one male was recorded when adding 1.0% ammonium acetate to GF-120 under high population (3.13 females; F = 4.27 and P = 0.012) and with GF-120 alone under low population (3.30 females; F = 21.88 and P <0.01). In contrast, the lowest number of attracted females per one male was recorded with the concentration of 2.0% under high population and when adding ammonium acetate with the concentration of 1.0% under low population; however, number of females was 1.69 and 0.61, respectively (Figure 6).
Figure 6.Sex ratio (as No. of females/ 1male) of attracted B. zonata flies to GF-120 enhanced by ammonium acetate at different concentrations under two levels (high and low) of pest population (In each population level; means have the same letter did not differ significantly at the probability of 0.05)
Discussion
It is likely that the ammonia emission rate is a significant determinant for the efficacy of candidate products, which is supported by the significantly higher catches of flies 39. Results here suggest that a change to GF-120 compassion is warranted. According to Yee 36, addition of ammonium carbonate and ammonium acetate enhanced the ability of GF-120 to attract R. pomonella under field conditions. Similarly, in this study, the ability of GF-120 bait to attract B. zonata flies was enhanced by adding di-ammonium phosphate, ammonium carbonate or ammonium acetate to the bait. The addition of these ammonium compounds (especially with concentrations of 3 and 4%) to the bait significantly attracted more B. zonata flies than bait alone. Pelz et al. 18 and Pelz-Stelinski et al. 19 found that fruit flies spent more time around GF-120 bait with additional ammonium acetate than GF-120 without it. They suggest that higher levels of ammonium acetate in GF-120 can increase arrestment of foraging flies. In another study, adding ammonium acetate to protein baits potentially increased the bait’s efficacy in attracting, monitoring and control of Ceratitis capitata (Wiedemann) 5. The current results are further confirmed by El-Metwally 40 who reported that addition of di-ammonium phosphate, ammonium carbonate and ammonium acetate to GF-120 bait improved its ability to attract C. capitata flies.
Among the ammonium compounds tested, di-ammonium phosphate improved the ability of GF-120 to attractive B. zonata flies the most, followed by ammonium carbonate and ammonium acetate. The same results were obtained for C. capitata40. Furthermore, Hemeida et al. 41 reported that di-ammonium phosphate was more effective in enhancing the ability of protein-biased baits (Buminal, Agrinal and Amadine) to attract B. zonata flies than ammonium acetate. In contrast, attractiveness of R. cingulata flies to GF-120 bait increased as concentrations of ammonium acetate increased compared to di-ammonium phosphate 19. But ammonium carbonate was more effective in improving the GF-120 bait to attract R. pomonella than ammonium acetate 36. This variation may be specific to fruit fly species or compound concentrations. Yee and Landolt 28 found that captures of R. pomonella flies in traps increased as concentrations of ammonia in lures increased from 0 to 29.3%. Limited improvement in attractiveness to R. pomonella also was reported for ammonium bicarbonate added to the protein hydrolysate bait, NuLure 42.
Results showed that attractiveness of highly concentrated treatments (especially the highest concentrations of di-ammonium phosphate) decreased after two days of hanging traps. This was explained by Yee 36 who documented that the lack of differences between GF-120 alone and GF-120 enhanced by ammonium carbonate or ammonium acetate was likely due to ammonia release rates from enhanced drops decreased quickly after sprays, so after a few days or even less time the enhanced GF-120 was the same as GF-120 alone in attractiveness. Ammonium acetate dissipates rapidly because of its high volatility 9. This volatilization is likely the cause of the loss of GF-120 attractiveness to the melon fly, BactroceracurcurbitaeCoquillett, observed within the first day after application. Further, the present results are consistent with the trends observed by Epsky et al. 43, 44. They mentioned that volatile attractant chemicals from Nulure/borax solution may be released at levels too high for effective fly capture when freshly prepared but are too low to have an effect on fly capture by 4-5 days after preparation. Vargas et al. 13 noted that aged baits (4 days-old) were as not attractive to C. capitata as were fresh baits.
The ability of GF-120 to attract B. zonata was found to be basically dependent on the ammonium compound, the concentrations, and pH with positive relationships. The degree of pH was positively affected by the concentration of added ammonium compound to GF-120. For example, each increase in the concentration of di-ammonium phosphate by one percent increased pH by 0.88, 0.71 and 0.86 degrees in fresh bait and at the end of treatment under high and low populations, respectively. These results are consistent with those of Bateman and Morton 45, Heath et al. 46, El-Gendy 32 and El-Metwally 40; they mentioned that the attracted fruit flies to ammonium compounds, food baits and GF-120 (that enhanced by ammonium compounds) were found to be strongly dependent on concentration of ammonium compound and therefore on pH level. Further, there were positive relationships between pH level of some protein-based baits and the attracted flies of B. zonata and C. capitata31. El-Gendy 32 added that borax makes the solution of ammonium compounds more alkaline and therefore increases release of ammonia from the bait solution (which increased the attracted B. zonata flies). Also, addition of 1-10% borax to 10% Nulure solution (as food attractant) increased pH level, resulting in increased captures of Anastrephasuspensa (Loew) and C. capitata in traps under field conditions 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. Mazor et al. 47 mentioned that the elevation of the pH of the liquid commercial baits, Buminal and Naziman, increased the efficacy of the latter for C. capitata but the increased stimulation could not be strictly correlated with the increased rate of ammonia release. The highest captures of C. capitata in McPhail traps occurred with Milhocina and borax adjusted to a pH of 8.5 33. In the present study, the highest attracted B. zonata flies were recorded when pH more than 7.00. On the other hand, B. zonata females were more responsive to the increase of pH and concentrations of ammonium compounds than males. Similar results were obtained by El-Metwally 40, who mentioned that females of C. capitata were more responded to the increase of pH in GF-120 preparations.
At the end of experiments, pH level decreased under high and low population levels; which resulted in decreased fly captures. These results are in agreement with El-Gendy 31, who mentioned that the properties of protein bait solutions in traps were changed along the elapsed time. Heath et al. 46 added that resultant pH affected by factors such as age of bait solution increased attraction of flies. According to the present study it may be affected by ecological factor such as air temperature degrees, relative humidity and/or population level of the attracted flies. Also, many authors showed through regression analysis that attraction of some ammonium compounds and food attractants decreased by time after treatment 24, 25, 26, 48. Epsky et al. 44 and El-Gendy 32 mentioned that there were no significant differences in pH levels of food baits and ammonium compounds over the time period of the test. Climatic and habitat differences may explain the inconsistency between the present results and others.
According to the relationships between concentrations of ammonium compounds and the resulted pH in the prepared solutions, there were positive relationships between them. The degrees of pH were more affected by the concentrations of ammonium compounds in fresh baits compared to those in baits at the end of experiment under field conditions. This may be attributed to the effects of ecological factors on the baits in the field. At the end of experiment under field conditions, pH varied from high to low pest population levels; whereas, the general pH-levels were higher in the high than low population experiment. This may be attributed to the high numbers of dead flies in traps in the high population experiment, which may have changed the chemical composition of the bait and therefore modified its pH level and therefore its attraction. This theory is agreement with that of Rousse et al. 30, who documented that the chemical composition of the bait may change because of dead flies, increasing its attraction to fruit flies, while living flies might emit attractive or repulsive volatiles.
The obtained results showed that GF-120 alone or enhanced by ammonium compounds attracted females more than males. These results are in agreement with those obtained by Yee 36 who reported that females of R. pomonella were more responsive to the GF-120 lures on traps than males. He added that the lures affected the sexes similarly in terms of relative responses. Also, many studies reported that ammonium compounds and protein-biased baits attracted more female than male flies 24, 25, 26, 29, 31, 32, 40, 41, 43, 49. Rousse et al. 30 showed that numerically more females than males of B. cucurbitae were caught in traps baited with food attractants especially when they were protein starved. Because fruit flies require a source of protein to complete egg maturation 50 this requirement is probably the main cause for the strong attraction of females towards decomposing proteinaceous substances 51.
The present results showed that the sex ratios of B. zonata (as number of attracted females per one male) obtained by GF-120 alone or enhanced by ammonium compounds were higher under low population level in comparison with high population level. This may be explained as follows: data in the high population level experiment were obtained in guava orchard at the end of the fruit ripening period (9-20 October 2017), where as in the low population level experiment they were obtained in navel orange orchard before the fruit ripening period (20-31 October 2017). Thus, many infested guava fruits were fallen under trees (as field observation) and most flies (females and males) were newly emerged; therefore both sexes of flies were attracted to food sources for nutrition. In navel orange orchard, females occurred as visitors searching for hosts to deposit their eggs. The present results are supported by El-Metwally 40, who mentioned that the sex ratios of attracted C. capitata to GF-120 enhanced with ammonium compounds tended to females in guava orchards more than those in navel orange orchards. Also, Pinero et al. 5 and Hemeida et al. 41 reported that adding di-ammonium phosphate, ammonium carbonate or ammonium acetate to a variety of protein baits and materials increased the numbers of attracted females of C. capitata and B. zonata in comparison with attracted males.
Conclusion
The ability of GF-120 to attract B. zonata flies can be enhanced by additing di-ammonium phosphate or ammonium carbonate at concentrations of 3 or 4%. In comparison with males, females of B. zonata were more responsive to the increase of pH. Attractiveness of GF-120 preparations is depending on pH; so, it can be used in chemical analysis for identification of new attractants from preferred bait formulations. Information regarding the effect of pH on the efficiency of different attractants in attracting fruit flies under various ecological situations should be studied.
Acknowledgments
The author wishes to thank Dr. Mostafa M. El-Metwally and Dr. Reda A. El-Sharkawy, Plant Protection Research Institute, Agricultural Research Center; for assisting in the field bioassay and determining pH in the present study.
References
- 1.JCM Loaiza.Céspedes CL (2007). Compuestos volatiles de plantas. Origen, emission efectos, análisis y aplicaciones al agro. , Revista Fitotecnia Mexicana 30, 327-351.
- 2.I S Joachim-Bravo, A N Guimarães, T C Magalhães. (2001) Influência de substâncias atrativas no comportamento alimentar e na preferência de oviposição deCeratitis capitata(Diptera:. , Tephritidae). Sitientibus, Série Ciências Biológicas 1, 60-65.
- 3.Kaspi R, P W Taylor, Yuval B. (2000) Diet and size influence sexual advertisement and copulatory success of males in Mediterranean fruit fly leks. , Ecological Entomology 25, 279-284.
- 4.Yuval B, Maor M, Levy K, Kaspi R, P W Taylor et al. (2007) Breakfast of champions or kiss of death? Survival and sexual performance of protein fed, sterile Mediterranean fruit flies. , Fl. Entomol 90, 115-122.
- 5.N D Epsky, P E Kendra, E Q Schnell. (2014) History and development of food-based attractants, In. T. Shelly, N. Epsky, E.B. Jang, J. Reyes-Flores, R.I. Vargas (eds.).Trappingandthedetection,controlandregulationoftephritidfruitflies:lures,area-wideprograms,andtradeimplications.Springer,The Netherlands 75-118.
- 6.J C Pinero, S K, T R Smith, A J Fox, R I Vargas. (2015) Ammonium acetate enhances the attractiveness of a variety of protein-based baits to femaleCeratitis capitata(Diptera:. , Tephritidae). J. Econ. Entomol 108, 694-700.
- 7.R I Vargas, S L Peck, G T McQuate, C G Jackson, J D Stark et al. (2001) Potential for areawide integrated management of Mediterranean fruit fly (Diptera: Tephritidae) with a braconid parasitoid and a novel bait spray. , J. Econ. Entomol 94, 817-825.
- 8.D S Moreno, R L Mangan. (2003) Bait matrix for novel toxicants for use in control of fruitflies(Diptera:Tephritidae), In:. Invasive Arthropods in Agriculture.SciencePublishers,Inc C. Schwalbe, (ed.): , Enfield, NH 333-362.
- 9.R J Prokopy, N W Miller, J C Pinero, J D Barry, L C Tran et al. (2003) Effectiveness of GF-120 fruit fly bait spray applied to border area plants for control of melon flies. , J. Econ. Entomol 96, 1485-1493.
- 10.R L Mangan. (2009) Effects of bait age and prior protein feeding on cumulative tie-dependent mortality ofAnastrephaludens(Diptera: Tephritidae) exposed to GF-120 spinosad baits. , J. Econ. Entomol 102, 1157-1163.
- 11.R L Mangan. (2014) History and development of food-based attractants.In:T. , The Netherlands 423-456.
- 12.D B Thomas, N D Epsky, C A Serra, D G Hall, P E Kendra et al. (2008) Ammonia formulations and capture ofAnastrephafruit flies (Diptera:. , Tephritidae). J. Entomol. Sci 43, 76-85.
- 13.R I Vargas, N W Miller, R J Prokopy. (2002) Attraction and feeding responses of Mediterranean fruit fly and a natural enemy to protein baits laced with two novel toxins, phloxine B and spinosad. , Entomol. Exp. Appl 102, 273-282.
- 14.J D Barry, Polavarapu S. (2004) Feeding activity and attraction of blueberry maggot (Diptera: Tephritidae) to protein baits, ammonium acetate, and sucrose. , J. Econ. Entomol 97, 1269-1277.
- 15.Mazor M, Gazit S, Reuven G, Efrat H. (2003) Unattractiveness of proteinaceous fruit fly baits to honey bees. , Crop Prot 22, 995-997.
- 16.D B Thomas, R L Mangan. (2005) Nontarget impact of spinosad GF-120 bait sprays for control of the Mexican fruit fly (Diptera: Tephritidae) in Texas. , J. Econ. Entomol 98, 1950-1956.
- 17.AgroSciences Dow. (2006) Supplemental Labeling, approved 06/05/06. GF-120 NF Naturalyte® Fruit Fly Bait. , Indianapolis, IN
- 18.K S Pelz, Isaacs R, J C Wise, L J Gut. (2005) Protection of fruit against infestation by apple maggot and blueberry maggot flies (Diptera: Tephritidae) using compounds containing spinosad. , J. Econ. Entomol 98, 432-437.
- 19.K S Pelz-Stelinski, L J Gut, Isaacs R. (2006) Behavioral responses ofRhagoletiscingulata(Diptera: Tephritidae) to GF-120 insecticidal bait enhanced with ammonium acetate. , J. Econ. Entomol 99, 1316-1320.
- 20.R I Vargas, Mau R F L, E B Jang, R M Faust, Wong L. (2008) The Hawaii fruit fly area-wide pest management program.In:. , O Koul, GW Cuperus and NC Elliott(eds):Area-wideIPM:TheorytoImplementation.CABIBooks,London,UnitedKingdom 300-325.
- 21.J L Kostarides. (2002) Integrated management strategies for control of cherry fruit flies,RhagoletiscingulataandRhagoletisfausta(Diptera:. Tephritidae). Ph. D. dissertation,MichiganStateUniversity,EastLansing,MI
- 23.W L Yee. (2006) Feeding history effects on feeding responses ofRhagoletisindifferens(Dipt., Tephritidae) to GF-120 and Nulure. , J. Appl. Entomol 130, 538-550.
- 24.Abd El-Kareim AI, Shanab L M, El-Naggar M E, Ghanim N M. (2008) Response of peach fruit fly,Bactrocerazonata(Saunders) (Diptera: Tephritidae) to some ammonium compounds as olfactory stimulants. , J. Agric. Sci. Mansoura Univ 33, 8965-8973.
- 25.S A Moustafa, N M Ghanim. (2008) Some ammonium compounds as olfactory stimulants for Mediterranean fruit fly,Ceratitis capitataWiedemann (Diptera:. , Tephritidae). J. Agric. Sci. Mansoura Univ 33, 8965-8973.
- 26.N M Ghanim, N F Abdel-Baky, M A Al-Doghairi, A H Fouly. (2014) Evaluation of some ammonium compounds as olfactory stimulants for ziziphus fruit fly,Carpomyaincompleta(Diptera: Tephritidae) in Christ’s thorn orchards at Qassim. , Saudi Arabia.J.PlantProt.andPath. MansouraUniv 5, 367-377.
- 27.M H Bayoumy, El-Metwally M M. (2017) Daily flight activity rhythms of the peach and Mediterranean fruit flies using sexual and olfactory attractants. DOI: 10.1556/038.52.2017.022. Acta Phytopathol. et Entomol.Hung.
- 28.W L Yee, P J Landolt. (2004) Responses of apple maggot (Diptera: Tephritidae) to ammonium hydroxide lures. , Can. Entomol 136, 139-142.
- 29.Heath R R, Epsky N D, Bloem S, Bloem K. (1994) pH effect on the attractiveness of a corn hydrolysate to the Mediterranean fruit fly and severalAnastrephaspecies (Diptera:. , Tephritidae). J. Econ. Entomol 87, 1008-1013.
- 30.Rousse P, P F Duyck, Quilici S, Ryckewaert P. (2005) Adjustment of field cage methodology for testing food attractants for fruit flies (Diptera:. , Tephritidae). Ann. Entomol. Soc. Am 98, 402-408.
- 31.I R El-Gendy. (2012) Evaluating attractency of some protein derivatives for the Mediterranean fruit fly,Ceratitis capitata(Wiedmann) and the peach fruit fly,Bactrocerazonata(Saunders). doi: 10.3923/ijar.2012. , Int. J. Agric. Res
- 32.I R El-Gendy. (2013) Response of peach fruit fly,Bactrocerazonata(Saunders) (Diptera: Tephritidae), to synthetic food-odor lures and extent the effect of pH on attracting the fly. , J. Entomol 10, 136-146.
- 33.Paiva P E B, Parra J R P. (2013) Hydrogen ionic potential (pH) of the attractant, trap density and control threshold forCeratitis capitata(Diptera: Tephritidae) on Hamlin oranges in Sao Paulo central region. , Brazil. Revista Brasileira de Fruticultura 35, 464-470.
- 34.I M White, Elson-Harris M. (1992) Fruit Flies of Economic Significance: Their Identification and Bionomics. International Institute of Entomology, CAB International , Wallingford (UK) 601.
- 35.R A Syed, M A Ghani, Murtza M. (1970) . Studies on Tephritid and their natural enemies in West Pakistan, 111.Dacuszonatus(Saunders): (Diptera: Tephritidae) Tech , Bull. Wel. Inst. Biol. Cont 13, 1-6.
- 36.W L Yee. (2007) Attraction, feeding, and control ofRhagoletispomonella(Diptera: Tephritidae) with GF-120 and added ammonia in Washington state. , Fl. Entomol 90, 665-673.
- 37.A H Hanafy, Awad A I, Abo-Sheasha M. (2001) Field evaluation of different compounds for attracting adults of peach fruit flyBactrocerazonata(Saunders) and Mediterranean fruit fly,Ceratituscapitata(Wied.) in guava orchards. , J. Agric. Sci. Mansoura Univ 26, 4537-4546.
- 39.D M Suckling, E P Jang, Holder P, Carvalho L, Stephens A E A. (2008) Evaluation of lure dispensers for fruit fly surveillance in New Zealand. , Pest Manag. Sci 64, 848-856.
- 40.El-Metwally M M. (2017) Enhancing the attraction efficiency of GF-120 for the Mediterranean fruit fly,Ceratitis capitata(Wied.) by adding some ammonium compounds. , J. Plant Prot. and Path., Mansoura Univ 8(11), 541-547.
- 41.I A Hemeida, N M Ghanim, Mosallam A M Z, H A EL-Shabrawy, B M Metwaa. (2017) Enhancement of some protein-based baits for attractingBactrocerazonata(Diptera: Tephritidae) by adding ammonium compounds. , Egypt. Acad. J. Biolog. Sci 10, 149-162.
- 42.Hendrichs J, Hendrichs M, Prokopy J, Prokopy R. (1990) How do apple maggot flies detect the presence of distant food?. , Massachusetts Fruit Notes 55, 3-5.
- 43.N D Epsky, Heath R R, J M Sivinski, Calkins C O, R M Baranowski et al. (1993) Evaluation of protein bait formulations for the Caribbean fruit fly (Diptera:Tephritidae). , Florida Entomologist 76, 626-635.
- 44.N D Epsky, P E Kendra, Heath R R. (2006) Response ofAnastrephasuspensato liquid protein baits and synthetic lure formulations. Fruit Flies of Economic Importance: From Basic to Applied Knowledge. Proc. of the 7thInternational Symposium on Fruit Flies of Economic Importance10-15September2006,Salvador , Brazil 81-88.
- 45.M A Bateman, T C Morton. (1981) The importance of ammonia in proteinaceous attractants for fruit-flies (family. , Tephritidae). Australian J. Agric. Res 32, 883-903.
- 46.Heath R R, Vazquez A, E Q Schnell, Villareal J, P E Kendra et al. (2009) Dynamics of pH modification of an acidic protein bait used for tropical fruit flies (Diptera:. , Tephritidae). J. Econ. Entomol 102, 2371-2376.
- 47.Mazor M, Gothilf S, Galun R. (1987) The role of ammonia in the attraction of females of the Mediterranean fruit fly to protein hydrolysate baits. , Ent. Exp. App 43, 25-29.
- 48.N M Ghanim. (2009) Studies on the peach fruit fly,Bactrocerazonata(Saunders). , (Tephritidae, Diptera). Unpublished Ph. D. Thesis, Mansoura Univ 121, pp..
- 49.El-Metwally M M. (2012) Response of the olive fruit fly,BactroceraoleaeRossi to some ammonium compounds and certain food attractants under field conditions in olive orchards. , J. Plant Prot. Pathol. Mansoura Univ 3, 491-502.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Egyptian Academic Journal of Biological Sciences F Toxicology & Pest Control (2024) OpenAlex
Hendawy M. A., Ahmed H. El-Afify, M. Bayoumy, R. Shreef, N. Ghanim et al. - Egyptian Academic Journal of Biological Sciences, F. Toxicology & Pest Control (2024) Semantic Scholar
N. M. Ghanim, M. El-Metwally - Acta Phytopathologica et Entomologica Hungarica (2019) Semantic Scholar
