Exploring the Correlation between Glucose and Apoptosis Levels in Stored Platelets
Abstract
Background and Objectives
Platelets are small, anucleate blood cells produced in the bone marrow, primarily involved in blood coagulation. Platelet concentrate is a vital blood product with extensive applications. However, its short lifespan and limited donor availability pose global challenges. This study aimed to follow the trend of platelets 5 during days of storage.
Material and Methods
We studied on 40 platelet bags and analyzed glucose levels, lactate dehydrogenase (LDH), bacterial culture, and apoptosis using flow cytometry with Annexin V-PI over three consecutive days (first, third, and fifth) post-blood collection. Data were analyzed using SPSS.
Results
No significant correlations were found between age, blood group, or gender and the variables studied. No bacterial growth was detected. Glucose levels decreased significantly from day 1 (382 mg/dl) to day 5 (298 mg/dl). The average platelet apoptosis increased significantly from 3.65% on day 1 to 9.06% on day 5. Significant correlations were observed between glucose levels and apoptosis on days 3 (p<0.05) and 5 (p<0.01). No correlation found between LDH and apoptosis or necrosis, although a significant relationship between necrosis and apoptosis was noted on day 5 (p=0.003).
Conclusion
These findings suggest that while demographic factors do not influence the studied variables, the significant decrease in glucose levels correlates with increased platelet apoptosis over time, highlighting potential metabolic interactions that warrant further investigation.
Highlights
1. The study revealed subtle variations in metabolic markers related to donor demographics, particularly gender and age. Understanding these differences can inform targeted donor selection strategies to optimize platelet quality.
2. A significant negative correlation was found between glucose levels and apoptosis rates, indicating that as glucose decreases, platelet viability declines. This relationship highlights the need for careful monitoring of glucose levels during storage to maintain platelet function.
3. Fluctuations in lactate dehydrogenase (LDH) levels were correlated with increasing rates of apoptosis, suggesting that LDH could serve as a valuable biomarker for assessing platelet quality throughout the storage period. This finding could lead to improved storage protocols and enhanced transfusion safety.
Author Contributions
Academic Editor: Anubha Bajaj, Consultant Histopathologist, A.B. Diagnostics, Delhi, India
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025Mohammad Kaboli, et al. 2025
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Since the first successful administration of platelets for the treatment of hemorrhagic disorders in 1910, platelet concentrate (PC) has become one of the most effective and essential pharmaceutical products. Consequently, the transfusion of platelet components is a critical method for treating various Donors, particularly those with hematologic and oncologic diseases 1.
Each unit of platelet concentrate is derived from a single unit of whole blood donated by one donor, with a volume of approximately 50 milliliters and a maximum storage time of three days. Although the minimum acceptable platelet count is defined as approximately 5.5 × 10^10 platelets in 75% of platelet concentrate units, these units typically contain about 7 × 10^10 platelets. The remaining volume of the platelet concentrate consists of an anticoagulant solution and donor plasma. Before transfusion, blood bank staff mix 5 to 8 units of platelet concentrate to produce a product containing approximately 3 to 6 × 10^11 platelets 2.
Platelet damage encompasses all injuries that threaten the morphology, structure, and function of platelets during storage. These detrimental effects can occur during blood collection or while preparing and processing the platelets. Additionally, during storage, metabolic activity within the platelets and red blood cells consumes nutrients in the bag, leading to the accumulation of harmful metabolites. Prolonged storage of platelets in blood bags results in increased lactate dehydrogenase (LDH) levels, decreased intracellular ATP, reduced glutathione, and elevated levels of membrane protein a-granule (GMP-140) 3. Furthermore, bacterial contamination can also negatively impact the quality of prepared platelets 4, 5, 6. So, we conducted a study to follow the trend of platelets 5 during days of storage.
Material and Methods
The study population consisted of blood donors at the blood transfusion centers in Gorgan, the capital of Golestan Province in northern Iran. The Donors recieved informed consent for participation in the investigation. This study was registered with the ethical code IR.GOUMS.REC.1403.316 at Golestan University of Medical Sciences.
During the implementation of the study, a number of platelet concentrate bags were assessed daily after undergoing quality control measures. Following standardized protocols, these bags were transferred from the blood transfusion organization to laboratories for biochemical, microbiological, hematological, and flow cytometry analyses.
Quality control tests for contamination and other parameters were carried out by the laboratory on the day of product preparation. The platelet concentrates were stored at 24 c in shaking incubators until testing, specifically on days 1, 3, and 5 post-collection. A total of 120 samples sent to the laboratory were tested on the same day they were collected.
Biochemical tests included the measurement of glucose and lactate dehydrogenase (LDH), both of which were conducted using Biorex kits (Iran) on a Mindray BS800 auto-analyzer. Microbiological assessments were performed using Blood agar and EMB culture media, with evaluations conducted after a 24-hour incubation period. Hematological analysis involved platelet counting using a MYTHIC 22 AL cell counter (Orphée). Additionally, flow cytometry was utilized to assess cell death, employing Annexin V and PI markers through kits from IQ Products, Netherlands, and executed on a BD FACS Calibur device from the United States, following the manufacturers' protocols.
Data analysis was performed using SPSS statistical software. To compare bacterial contamination, biochemical factors, and cell death rates in platelet concentrates, as well as the age and gender of the donors, the Chi-square test was employed. A p-value of less than 0.05 was considered statistically significant.
Results
In this study, 40 blood donors (10 from each of the four main ABO blood groups) participated, comprising 25 males (62.5%) and 15 females (37.5%). The mean age of the participants was 40.61 years, with an average age of 41.81 years for females and 39.84 years for males. Descriptive statistics for the variables, irrespective of gender and blood group, are presented in Table 1. A total of 120 tests were conducted on the donated platelets from the 40 participants.
Table 1. Descriptive Statistics of the Study Population| Min | Max | Mean | SD | |
| Age | 21 | 57 | 40.45 | 10.60 |
| Glucose Day 1 | 275 | 500 | 382.05 | 49.10 |
| Glucose Day 3 | 121 | 458 | 341.55 | 66.50 |
| Glucose Day 5 | 64 | 443 | 298.13 | 80.66 |
| LDH day 1 | 358 | 1017 | 805.1 | 171.09 |
| LDH day 3 | 331 | 1021 | 832.9 | 153.66 |
| LDH day 5 | 384 | 1000 | 774.75 | 172.37 |
| Apoptosis Day 1 | 0.3 | 20 | 3.843 | 4.13 |
| Apoptosis Day 3 | 0.3 | 13 | 5.949 | 3.48 |
| Apoptosis Day 5 | 1.1 | 25 | 9.062 | 5.93 |
| Necrosis Day 1 | 0 | 0.8 | 0.079 | 0.18 |
| Necrosis Day 3 | 0 | 2 | 0.339 | 0.55 |
| Necrosis Day 5 | 0 | 2 | 0.297 | 0.57 |
The analysis of all samples collected on days one, three, and five indicated that the platelet concentrate bags were suitable for storage. To investigate the effect of gender on the donated platelet samples, all research variables were compared between the two groups. The results showed no significant differences between the two groups (Table 2). Furthermore, our findings revealed no statistically significant correlation between age and any of the variables among the 40 participants and the 120 tests conducted. Additionally, microbiological cultures of the platelet samples showed no bacterial growth, with all 120 samples testing negative for microbial contamination.
Table 2. Comparison of Variable Ranges Based on Gender| Gender | Male | Female | pValue |
| Glucose day 1 | 387±48 | 372±49 | 0.352 |
| Glucose day 3 | 353±56 | 321±78 | 0.144 |
| Glucose day 5 | 303±92 | 289±58 | 0.589 |
| LDH day 1 | 767±187 | 867±121 | 0.074 |
| LDH day 3 | 800±165 | 887.33±116 | 0.083 |
| LDH day 5 | 769±161 | 783±195 | 0.817 |
| Apoptosis day 1 | 4.01±4.45 | 3.56±3.67 | 0.747 |
| Apoptosis day 3 | 6.11±3.71 | 5.66±3.13 | 0.702 |
| Apoptosis day 5 | 9.608±6.47 | 8.18±5.03 | 0.745 |
| Necrosis day 1 | 0.1±0.21 | 0.047±0.106 | 0.37 |
| Necrosis day 3 | 0.267±0.48 | 0.46±0.65 | 0.292 |
| Necrosis day 5 | 0.333±0.58 | 0.24±0.56 | 0.625 |
When examining the impact of blood groups on the donated platelet samples, all research variables were compared across the four blood groups. Our results indicated no significant differences in the examined variables among the different blood groups.
Given the lack of impact from gender and blood groups on the results, all subsequent analyses were performed on the variables as a whole (Table 3). As expected, glucose levels in the samples significantly declined during the storage period over the days assessed. The average glucose levels in the platelet bags on days 1, 3, and 5 were mg/dl 382, 342, and 298, respectively. Contrary to expectations, LDH levels did not show significant differences except between days three and five (p=0.041). The average LDH levels in the platelet bags on days 1, 3, and 5 were U/L 805, 833, and 775, respectively.
Table 3. Correlation between Variables within Groups| Mean | SD | pValue | |
| Glusos. Day 1 | 382 | 49 | 0.001 |
| Glusos. Day 3. | 342 | 66 | |
| Glusos. Day 1 | 382 | 49 | 0.001 |
| Glusos. Day 5 | 298 | 81 | |
| Glusos. Day 3. | 342 | 66 | 0.001 |
| Glusos. Day 5 | 298 | 81 | |
| LDH.Day 1 | 805 | 171 | 0.209 |
| LDH.Day 3 | 833 | 154 | |
| LDH.Day 1 | 805 | 171 | 0.353 |
| LDH.Day 5 | 775 | 172 | |
| LDH.Day 3 | 833 | 154 | 0.041 |
| LDH.Day 5 | 775 | 172 | |
| Apoptosis. Day 1 | 3.63 | 3.96 | 0.008 |
| Apoptosis. Day 3 | 5.95 | 3.48 | |
| Apoptosis. Day 1 | 3.84 | 4.13 | 0.001 |
| Apoptosis. Day 5 | 9.06 | 5.93 | |
| Apoptosis. Day 3 | 5.95 | 3.48 | 0.018 |
| Apoptosis. Day 5 | 9.01 | 6.01 | |
| Necrosis.Day 1 | 0.08 | 0.18 | 0.013 |
| Necrosis.Day 3 | 0.34 | 0.55 | |
| Necrosis.Day 1 | 0.08 | 0.18 | 0.025 |
| Necrosis.Day 5 | 0.3 | 0.57 | |
| Necrosis.Day 3 | 0.34 | 0.55 | 0.737 |
| Necrosis.Day 5 | 0.3 | 0.58 |
Furthermore, the analysis of apoptosis indicated significant differences across the days studied, with average apoptosis levels in the platelet bags showing an increasing trend of 3.65%, 5.95%, and 9.06% on days 1, 3, and 5, respectively. The necrosis results indicated no significant statistical difference between days three and five; however, significant differences were observed between days one and three, as well as between days one and five. The average necrosis rates in the platelet bags were 0.08%, 0.34%, and 0.30% on days 1, 3, and 5, respectively. As expected, the percentage of viable platelets decreased over time, with average values of 95.93%, 93.67%, and 90.75% on days 1, 3, and 5, respectively (Figure 1 and Figure 2).
Figure 1.Flow Cytometry Analysis of Platelet on Days 1, 3, and 5 Post-Collection. An example of the flow cytometry results on days one, three, and five was conducted to determine the rates of cell death. Quadrant Q1 represents Annexin-V positive and PI negative cells, Q2 represents Annexin-V positive and PI positive cells, Q3 represents Annexin-V negative and PI positive cells, and Q4 represents Annexin-V negative and PI negative cells.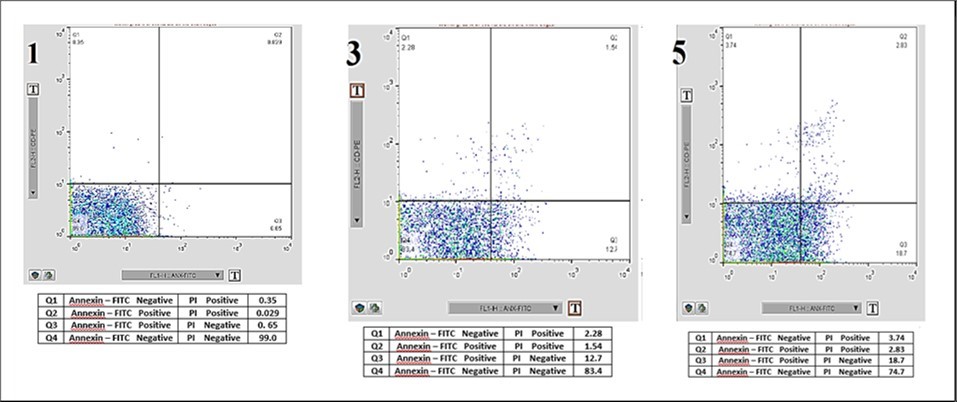
Figure 2.Comparative Chart of Apoptosis Rates in Platelets Stored on Days 1, 3, and 5 Post-Collection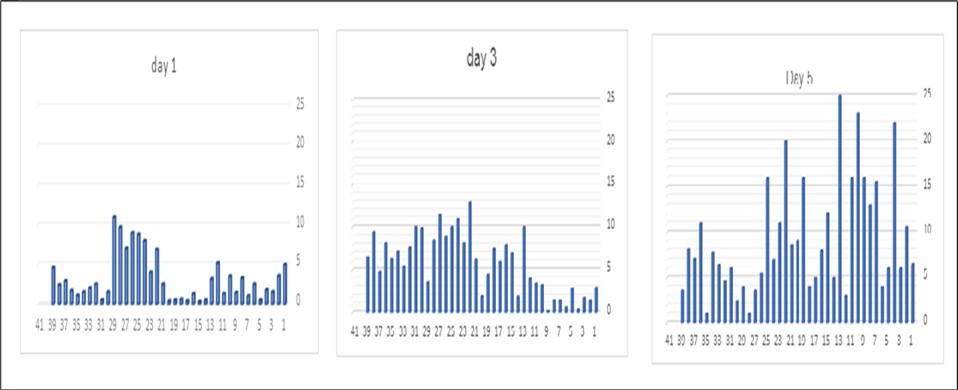
The analysis of correlations between variables indicated a significant relationship on day one between the decrease in glucose levels and the increase in LDH levels (p=0.017), while no other significant relationships were observed. On day three, a strong correlation was noted between reduced glucose and increased LDH levels (p=0.000), as well as a statistically significant relationship between decreased glucose and increased apoptosis (p=0.026). No significant correlations were found in other instances. On day five, no correlation was observed between glucose and LDH levels; however, both apoptosis (p=0.018) and necrosis (p=0.001) exhibited significant relationships with decreasing glucose levels, indicating an increase. Additionally, a statistically significant relationship was found between necrosis and apoptosis on day five (p=0.003), while no significant correlations were observed in other cases.
Discussion
The availability of platelet concentrates is limited due to the formation of platelet storage lesions (PSL) and the risk of bacterial contamination. Platelet storage lesions are a set of biochemical, structural, and functional changes that occur from the time of blood collection until the blood is transfused to the recipient in the platelet bag. Understanding PSLs is crucial for developing interventions that extend the shelf life of platelet concentrates to improve access and reduce waste. PLS occures during handling and the use of high-speed centrifugation during the preparation of platelet concentrates make conditions unsuitable for maintaining high-quality platelets. Platelet storage lesions begin with platelet activation, fragmentation, and biochemical release. During the storage of platelet concentrates at room temperature, increased glycolysis and decreased mitochondrial function lead to reduced glucose levels, lactate accumulation, and acidification of the product. Disruption in the production of adenosine triphosphate (ATP) reduces the capacity of platelets to perform energy-intensive processes. The microparticles formed due to storage in the platelet bag are associated with increased platelet aggregation and immune system regulation 7.
The results of the present study showed that there is no statistically significant relationship between age, gender, and blood type with platelet apoptotic death in platelets concentrate bags. Among the most important findings of this study is the expected and statistically significant reduction of glucose present in the bags of platelet concentrates over time from the first day to the fifth day. The decrease in glucose levels consumed in the bag by platelets indicates energy production in the cells. The average levels of these changes were from 382 on the first day, to 342 on the third day, and finally 298 mg/dl on the fifth day. However, considering the results of other previous studies, including the study by Aibibula and colleagues in 2018 8, which showed that the main pathway for ATP energy production by platelets is glycolysis and glucose consumption, this is not unexpected.
In the study by Sandgren and colleagues (32), it was also shown that during the storage of platelets with plasma from the first to the ninth day, glucose levels decrease, and compared to glucose levels in platelets with the commercial preservation solution InterSol, the decrease is less.
On the other hand, in our study, a statistically significant relationship was observed between the decrease in glucose levels in the bags of platelet concentrates on the third (0.026) and fifth (0.018) days with increased apoptosis, such that this relationship slightly increases on the fifth day, but on the first day, there is no statistical relationship (0.479), indicating the importance of this finding. Considering that with the decrease in glucose levels in the environment, platelets utilize alternative pathways such as fatty acid oxidation for their energy supply; this issue may be significant in increasing the survival of platelets.
Thus, by studying the mechanisms effective in inducing or preventing apoptosis in platelets, the percentage of viable platelets can be increased. However, the question arises whether platelets whose lifespan has been artificially extended by methods have the biological capabilities for their normal functions in the recipient's body 9. For example, using cold conditions in the preparation or storage of platelets can extend their lifespan, but studies in this area have shown that such platelets do not perform adequately and are quickly eliminated after entering the recipient's body 10. Platelets have a short life span in circulation before being cleared by the reticuloendothelial system: 8 to 10 days in humans. This is not due to their anucleated nature, as red blood cells in humans have a lifespan of about 3 months. The role of members of the BCL-2 family and caspases in platelet apoptosis was first reported 20 years ago; only in the last decade has the central importance of the intrinsic apoptotic pathway for their survival become clear. Our study results showed that the average level of apoptosis in platelet concentrates significantly increased over time, with values of 3.65%, 5.95%, and 9.06% on days 1, 3, and 5, respectively. Platelet apoptosis is associated with various events, including mitochondrial inner membrane depolarization, increased expression, activity, and translocation of Bak and Bax proteins on the mitochondrial surface, release of cytochrome C into the cytosol, caspase activity, breakdown and degradation of cytoskeletal proteins, and increased expression of phosphatidylserine on the outer membrane surface of platelets. Mitochondria play a crucial role in maintaining the integrity and function of platelets during storage by producing ATP. Decreased oxygen tension within platelet bags is a trigger for increased production of reactive oxygen species (ROS) from mitochondria. In many studies, ROS has been introduced as an indicator of platelet activity and an inducer of apoptosis, and simultaneous increases in ROS and P-selectin during storage in platelet products have been reported. Platelets may become activated during collection, preparation, and storage. Evidence shows that activated platelets express phosphatidylserine. The expression of phosphatidylserine on the surface of platelets provides a pro-coagulant membrane for clotting factors and leads to the formation of prothrombin complexes and consequently thrombus formation. Phosphatidylserine-expressing platelets in circulation are recognized and cleared by phagocytes, macrophages, and dendritic cells. In fact, increased expression of phosphatidylserine on the surface of platelets is a signal for clearance by phagocytes 11.
One notable finding was the lack of correlation between LDH changes and apoptosis during days 1, 3, and 5 after the storage of platelet concentrates. Since the increase of the intracellular enzyme LDH in the plasma of platelet concentrate bags is associated with cell degradation and can be related to conditions accompanied by stress and impact on the bags, this indicates the proper collection and storage conditions of this product in the Golestan Blood Transfusion Center. Our results regarding the increase in LDH levels are quite similar to the previous study by Dr. Yari and colleagues in 2011 12, which showed that the level of this enzyme increased during the days 2, 4, and 7 of platelet storage. Their study results indicated that the use of the Composol solution as a preservative in the platelet bag significantly and meaningfully reduces the increasing changes in LDH.
Although the continued increase in LDH levels until the last day of examination in that study continued in 54 donors, our results on the fifth day showed a decrease in the average level of this enzyme in 40 donors. Similarly, the results of the study by Diehim and colleagues in 2020 on 10 platelets concentrate bags on days 1, 3, 5, and 7 showed that the levels of LDH increased linearly. They demonstrated that the increase in lactate dehydrogenase in platelets exposed to L-carnitine was significantly lower 13.
The results of microbiological cultures showed that no bacterial growth was observed in any of the 120 samples examined. The risk of bacterial contamination exists from the time blood is collected from the donor until various stages of blood product preparation. Additionally, in our study, no correlation was observed between necrosis and LDH changes. The average level of necrosis in the samples examined was very low, at 0.08%, 0.34%, and 0.30% on days 1, 3, and 5, respectively.
Another significant finding of this study was the existence of a statistical relationship between apoptosis and necrosis on the fifth day of storage of this blood product. While there is a large difference between these two variables on the first day of storage of the concentrate bag, this difference decreases on the third day and approaches statistical significance, and on the fifth day, it becomes significantly meaningful. This finding also provides a suitable basis for further studies and may relate to the natural process of apoptotic death and the accumulation of waste and unwanted substances such as lactate, which increases necrosis. A similar statistical change over time was observed between the two variables FBS and LDH, where the correlation increased on days one and three until it became very strong, but was lost on the fifth day, which could be an interesting target for further studies in the future.
The use of platelet additive solutions or PAS (Platelet Additive Solutions) to improve the storage conditions of platelet concentrates and reduce the risk of adverse transfusion reactions can be very effective. Common components found in PAS include sodium chloride, trisodium citrate, sodium acetate and/or sodium phosphate, D-mannitol, potassium chloride, and magnesium chloride/sulfate. The significant advantage of using PAS is that it allows platelets to be stored in minimal plasma volume, significantly reducing immunological responses (such as isoagglutinins in plasma), allergic reactions, and overload from simultaneous plasma transfusion. PAS contains electrolytes that improve platelet storage and are optimized to maximize platelet shelf life and consequently their quality 14. Gupta and colleagues analyzed the platelet count, platelet factor 3, lactate dehydrogenase (LDH), pH, glucose, and platelet aggregation of 30 random donor platelet samples. Once again, the results indicate that platelets can extend their shelf life to 7 days without the risk of bacterial contamination and loss of functional quality. They have shown that the quality of platelets stored in PAS is superior to that of platelets stored in plasma 15.
Conclusion
Based on the obtained results, it is suggested that future studies consider criteria for assessing platelets viability. Given the lack of significant changes in LDH levels within the bag, it appears that platelet loss does not occur through physical elimination. Instead, platelets undergo programmed cell death (apoptosis) despite being present in the concentrate bag, rendering them ineffective for their role after transfusion to the patient.
In this regard, it is recommended that during the storage of platelet concentrates and within the time frame up to the fifth day, platelet differentiation markers related to pre- and post-activation of these cells be examined. This would allow for a more thorough investigation of the various factors affecting the quality of donated platelets.
Additionally, it is suggested to study the effects of platelet additive solutions (PAS) and each of the components within these solutions in laboratory conditions on platelets and their influencing variables, such as activity, apoptosis, glucose levels, and LDH, and to compare these findings with the results of this study.
Acknowledgment
This article is derived from the thesis, completed as part of the requirements for the degree of Doctor of Medicine at Golestan University of Medical Sciences (GOUMS). The research presented here reflects the findings and insights gained during the author's training as a general physician.
Funding
The author(s) received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Panova-Noeva M, Meijden P E van der, Cate Ten, H. (2019) Clinical applications, pitfalls, and uncertainties of thrombin generation in the presence of platelets. , Journal of clinical medicine 9(1), 92.
- 2.Tynngård Nahreen. (2009) Preparation, storage and quality control of platelet concentrates. , Transfusion and Apheresis Science 41(2), 97-104.
- 3.Lippa S, Mores N, Aureli V, Fagiolo E. (1987) Biochemical and functional changes of platelet stored for transfusional use. Folia Haematologica. , Leipzig, Germany: 114(5), 680-685.
- 4.J H Levy, Neal, J H Herman. (2018) Bacterial contamination of platelets for transfusion: strategies for prevention. , Critical Care 22, 1-8.
- 5.P M Ketter, Kamucheka R, Arulanandam B, Akers K, A P Cap. (2019) Platelet enhancement of bacterial growth during room temperature storage: mitigation through refrigeration. , Transfusion 59, 1479-1489.
- 6.Baron B, K M Grech. (2021) Storage of platelet concentrates-looking beyond standard parameters for prolonged storage.
- 7.Ng M S Y, J P Tung, J F Fraser. (2018) Platelet storage lesions: what more do we know now?. Transfusion medicine reviews. 32(3), 144-154.
- 8.Aibibula M, K M Naseem, R G Sturmey. (2018) Glucose metabolism and metabolic flexibility in blood platelets. , Journal of Thrombosis and Haemostasis 16(11), 2300-2314.
- 9.Sandgren P, Mayaudon V, Payrat J M, Sjödin A, Gulliksson H. (2010) Storage of buffy-coat-derived platelets in additive solutions: in vitro effects on platelets stored in reformulated PAS supplied by a 20% plasma carry-over. Vox Sang. 2, 415-22.
- 10.J P Mack, Miles J, Stolla M. (2020) Cold-stored platelets: review of studies in humans. , Transfusion Medicine Reviews 34(4), 221-226.
- 11.Kiani Nodeh F, Ghasemzadeh M, Hosseini E. (2021) Apoptosis as a marker of platelet storage lesion. , Sci J Iran Blood Transfus Organ 18(4).
- 12.Maghsudlu. (2011) Iranian journal of pediatric hematology and oncology. , EVALUATION OF BIOCHEMICAL PARAMETERS OF PLATELET CONCENTRATES STORED IN PLASMA OR IN A PLATELET ADDITIVE SOLUTION (COMPOSOL)}, H. A. Izadpanahi and Yari and MR Khorramizadeh 1, 83-8.
- 13.Dahaj F, M, H N, M R Deyhim. (2021) C release during storage. , Journal of Thrombosis and Thrombolysis 51, 277-285.
