Metal Metalloproteinase 2 is Differentially Regulated by the Interplay between Glucose and Insulin
Abstract
Pre-adipocytes are the precursors with the potential to make new fat cells during adipose tissue expansion. Nevertheless, the pre-adipocytes behaviors, and their possible roles in energy homeostasis have long been overlooked. Our previous study implicates that interleukin-4 (IL-4) plays a positive metabolic role by promoting insulin sensitivity and inhibiting lipid accumulation. Besides, abundant evidence shows the involvement of matrix metalloproteinase-2 (MMP-2) in the process of adipose tissue expansion. The present study aimed at examining the cross talk among glucose, insulin and IL-4 on regulating MMP-2 expression and activity in 3T3-L1 pre-adipocytes. Effects of insulin and/or IL-4 on MMP-2 expression and activity were examined in pre-adipocytes under euglycemic or hyperglycemic environment by RT-PCR and gelatin zymography, respectively. Our results revealed that glucose level is a pre-requisite for pre-adipocytes responding to insulin and/or IL-4 treatment. In high glucose-containing environment, short-term acute insulin treatment (AI) and long-term chronic insulin exposure (CI) showed opposite regulation to MMP-2 expression and activity. Interestingly, the dominant MMPs regulatory role of CI under euglycemic condition was attenuated in cells exposed to high glucose concentration. Our results suggest pre-adipocytes may participate in the process of increasing adiposity, diabetic onset and diabetic complications through ECM alterations resulted from the insulin- and/or glucose- mediated changes of MMP-2 activity. The present study uncovers novel observations regarding pre-adipocytes behaviors.
Author Contributions
Academic Editor: Ronald D Fritz, PepsiCo, Inc., Purchase, NY, United states.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Jen-Ning Tsai, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Obesity is a key risk factor leading to metabolic abnormalities such as type 2 diabetes mellitus (T2DM), and has become a global health problem with rapid-growing incidence.1 Adipose tissue is composed of the most abundant adipocytes and other cells, such as pre-adipocytes, stromal-vascular cells, and endothelial cells. Coordinated cellular processes, including adipocyte hyperplasia, hypertrophy and proliferation, as well as the differentiation of pre-adipocytes to form new adipocytes (adipogenesis), are required for adipose tissue expansion.2,3 The extracellular matrix (ECM) must tackle dramatic challenges to meet the needs for morphological changes of pre-adipocytes transforming to mature adipocytes, and the enlargement of adipocytes during the process of increasing adiposity. Accordingly, changes of ECM components are crucial for the growth of the fat mass during the progression from lean to obese state.4,5
Matrix metalloproteinases (MMPs) are a family of enzymes that play crucial rolesin connective tissue remodeling by degrading basementmembrane and surrounding ECMcomponents in various physiological and pathological situations. The proteolytic activities of adipocytes-derived MMP-2 are induced during adipogenesis,6 which indicates that MMP-2 is an important regulator of adipocyte differentiation. Moreover, MMP-2 is strongly induced in the enlarged adipose tissue,7,8 suggesting MMPs could increase matrix plasticity and thereby facilitate adipose tissue remodeling and hypertrophy.
Evidence demonstrates that enhanced production of collagen is a key event in the development of diabetic glomerular ECM abnormalities. ECM proteins are accumulated within the renal interstitium in progressive tubular atrophy, and MMPs are suggested to participate in the process of concurrent reduced degradation and the enlargement of mesangial area during the pathogenesis of diabetic nephropathy.9,10,11 Therefore, the MMP-mediated ECM alterations are required both for the processes of adipose tissue expansion and the development of diabetic related complications.
Although the role of MMP-2 expression and activities in adipogenesis and diabetic complications are the study focus with abundant evidence, data concerning MMP-2 regulation in pre-adipocytes, which may play crucial roles for ECM remodeling during fat mass increment, are scarce. Particularly, the responses of pre-adipocytes to insulin, the major mediator maintaining energy homeostasis, and/or other external stimuli have long been overlooked.
Interleukin-4 (IL-4) is a pleiotropic anti-inflammatory cytokine which participates in subtle regulation of immune responses by acting on a wide variety of target cells.12 We previous identified the significant associations between interleukin-4 (IL-4)/IL-4 receptor genotypes and metabolic parameters such as high density lipoprotein-cholesterol.13,14 We also demonstrated that IL-4 improves insulin sensitivity and glucose tolerance while inhibits lipid accumulation in fat tissues.15 Moreover, IL-4 harbors anti-lipogenic ability by suppressing adipogenesis and promoting lipolysis in adipocytes,16,17,18 and boosts insulin-induced energy deposits by enhancing glucose uptake in hepatocytes.19 The above results strongly suggest that IL-4 regulates energy metabolism by modulating adipocyte behaviors. In the present study, the putative regulation of IL-4 and its interaction with insulin to MMP-2 in pre-adipocytes were examined for further elucidating the roles of IL-4 in adipose tissue biology. Hopefully, this study provides information regarding pre-adipocytes’ behaviors for understanding these adipocyte precursors, and uncovering their roles during the process of weight gain and adipose expansion.
Materials and Methods
Cell Culture and Adipogenesis
3T3-L1 pre-adipocytes were maintained in DMEM containing 10% calf serum (Hyclone Laboratories, South Logan, Utah, USA). For adipogenesis,16,17,18 2-day post-confluent cells were induced to differentiate by a standard cocktail composed of 0.5 mM 3-isobutyl-methylxanthine, 1 μM dexamethasone, and 10 μg/ml insulin in 10% FBS for 2 days. The cells were then cultured in DMEM supplemented with 10% FBS and 5 μg/ml insulin. For low- (LG) and high- (HG) glucose treatments, cells were cultured in medium containing 1 g/L and 4.5 g/L glucose respectively for 48h. For insulin and IL-4 treatment, during the final 18 hr and 30 min of the 48 hr incubation in LG or HG, cells were exposed to either 1 nM (CI) or 100 nM (AI) insulin with 100 nM IL-4.
RNA Extraction and RT-PCR
Total RNA was isolated using TRIZOL reagent (Life Technologies, Gaithersburg, MD). Briefly, cDNA was synthesized using 5 μg RNA, 200 pmol oligo dTprimer and 5× MMLV RT. Three μL of first-strand cDNA was amplified using target sequence-specific PCR primer sets (MMP-2: 5’-TGTGTCTTCCCCTTCACTTT-3’ and 5’-GATCTGAGCGATGCCATCAA-3’; GAPDH: 5’-TATGACAACTCCCTCAAGAT-3’ and 5’-AGATCCACAACGGATACATT-3’). All RT-PCR reactions were carried out with BIO-RAD PCR iCYCLER instrument. Amplified products were identified by electrophoresis on 2% agarose gel.
Analysis of MMP-2 Activities
MMP-2 activities were detected using gelatin zymography as described.20,21 In brief, cell culture media containing 20 μg of total proteins were loaded into each well on 10% gelatin zymography gels. After electrophoresis, the gels were rinsed twice in 2.5% Triton X-100 and incubated overnight (16 h) in substrate buffer containing 50 mM Tris-HCl and 5 mM CaCl2. The activated gels were stained by Coomassie blue R-250, and followed, by destaining.
Results
Regulation of IL-4/insulin on MMP-2 mRNA in 3T3-L1 Pre-Adipocytes (Figure 1)
Regulation of MMP-2 expression in pre-adipocytes by IL-4 and insulin was investigated for examining the response of pre-adipocytes to external stimuli. 3T3-L1 pre-adipocytes were maintained in standard media containing 4.5 g/L glucose (high glucose, HG), then MMP-2 mRNA was measured after the cells were treated with IL-4 or insulin. The results showed that MMP-2 was slightly elevated in the presence of IL-4 or insulin treatment (Figure 1, lanes 1-4).
Figure 1.Regulation of MMP-2 mRNA by IL-4 and insulin in pre-adipocytes.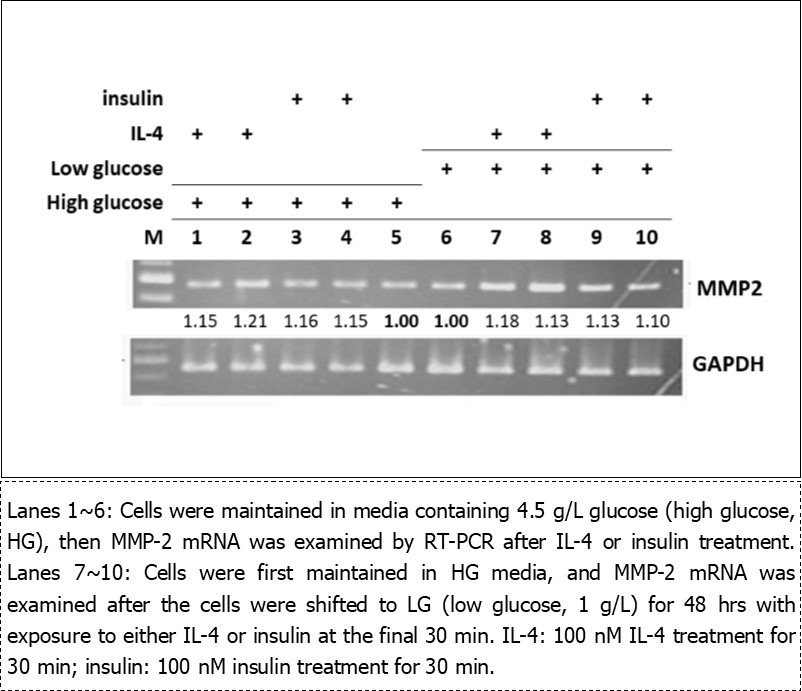
Glucose in regular culture media is equivalent to physiological HG concentration. We next explored if physiological euglycemic glucose (low glucose, LG; 1 g/L) would deviate pre-adipocytes’ behaviors and thus mediate MMP-2 expression in response to IL-4 or insulin. Cells were first maintained in HG, and MMP-2 mRNA was examined after the cells were shifted to LG for 48 hrs with either IL-4 or insulin treatment at the final 30 min. Likewise, MMP-2 mRNA was moderately increased under IL-4 or insulin treatment (lanes 7-10). The above results suggest that the environmental glucose does not significantly modulate MMP-2 expression in cells responding to IL-4 and insulin.
Effects of insulin on MMP-2 mRNA in 3T3-L1 Pre-Adipocytes (Figure 2)
Hyperinsulinemia resulted from the insulin resistance and/or inflammation is linked to adverse cardiovascular diseases.21,22,23 In this context, it is intriguing to examine if pre-adipocytes response differentially to different insulin concentrations.
Pre-adipocytes were maintained in HG, then MMP-2 mRNA was analyzed after the cells were exposed to either short-term (100 nM for 30 min, AI) or long-term (1 nM for 18 hr, CI) insulin with IL-4 treatment. IL-4 (lane 1) and AI (lane 3) did not cause dramatic changes of MMP-2 (lane 1), whereas, CI significantly suppressed MMP-2 (lane 2). Intriguingly, while CI showed a dominant role to downregulate MMP-2 (lane 4) under combined CI/IL-4 treatment, AI did not significantly alter MMP-2 expression (lanes 5-6). The above data indicate CI and AI exert differential regulation to MMP-2: CI takes the dominant role to suppress MMP-2 expression while short-term insulin treatment does not significantly modulate MMP-2 in pre-adipocytes.
Regulation of IL-4/insulin on MMP-2 Actvities in 3T3-L1 pre-Adipocytes
Possible alterations of MMP-2 activities resulted by IL-4 or insulin treatment in pre-adipocytes were subsequently investigated. Cells were maintained in HG, exposed to IL-4 and/or insulin, then MMP-2 activities were analyzed (Figure 3). IL-4 and AI did not cause prominent alterations of MMP-2 activities (lanes 1&3), while CI significantly up-regulated MMP-2 activities (lane 2). Notably, CI had a dominant feature under combined treatment (lane 4) although CI inhibited MMP-2 mRNA (Figure 2, lane 4). It indicates that CI reciprocally regulates MMP-2 by promoting MMP-2 activities while inhibiting MMP-2 expression, either in CI treatment alone or with concurrent IL-4 exposure.
Figure 2.Effects of insulin and IL-4 on MMP-2 mRNA in pre-adipocytes under high glucose environment.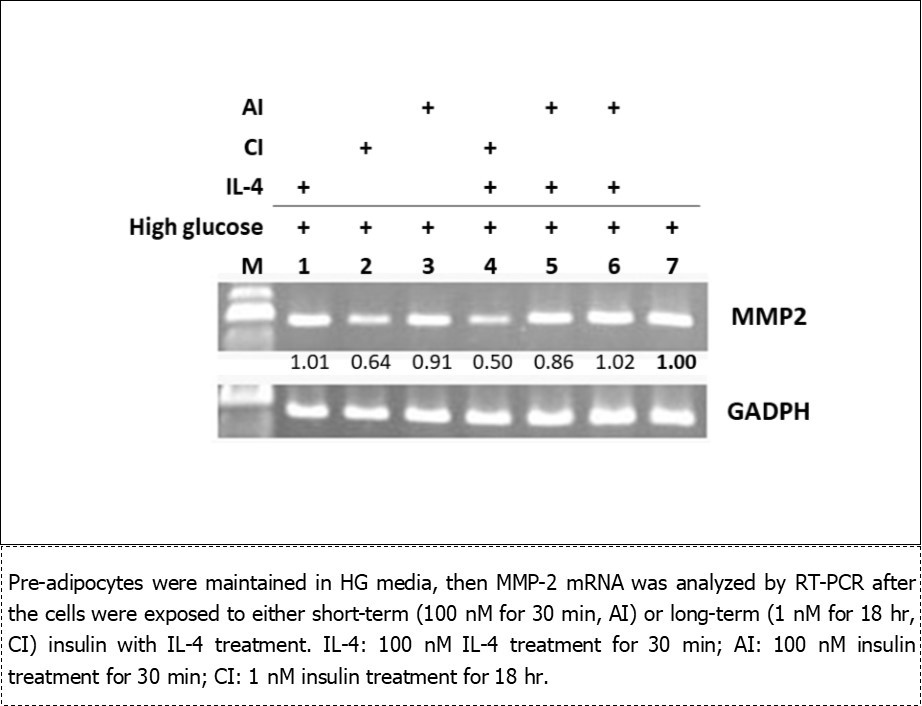
Figure 3.Effects of IL-4 and insulin on MMP-2 activities in pre-adipocytes.
MMP-2 activities in pre-adipocytes with IL-4 and/or insulin treatment under different glucose-containing environments were subsequently examined. Cells were first maintained in LG, and MMP-2 activities were analyzed after cells were exposed to either AI or CI with IL-4 treatment in LG or by shifting to HG for further 48 hrs (Figure 4). MMP-2 activities were slightly increased in cells after shifting to HG (lane 2). Under LG condition, CI significantly down-regulated MMP-2 activities (lane 3) while AI slightly promoted MMP-2 activities (lane 4). Interestingly, the significantly CI-downregulated MMP-2 activities in LG were recovered after the cells were shifted in HG (lane 5). However, no prominent changes were observed in AI treatment. It suggests that environmental glucose is a dominant factor overriding insulin for regulating MMP 2 activities, rather than MMP-2 expression, in pre-adipocytes.
Figure 4.Interactions between IL-4 and insulin on MMP-2 activity in pre-adipocytes under different glucose environment.
Taken the above observations together, it indicates that CI harbors negative regulatory effects on MMP-2 mRNA but promotes MMP-2 activities in HG. Interestingly, the CI inhibitory effects on MMP-2 activities in LG are abolished when cells are exposed to hyperglycemia. These results support that glucose is the critical factor which determines pre-adipocytes’ behaviors in response to chronic insulin treatment. The pre-adipocytes seem relatively less-sensitive and “reluctant” to respond to external stimuli in hyperglycemic condition.
Regulation of IL-4/insulin on MMP-2 mRNA and Activities in Mature Adipocytes
We next explored if the MMP-2 regulatory machinery in fully differentiated mature adipocytes showed similar responses to IL-4 and/or insulin as the scenario in pre-adipocytes. MMP-2 mRNA was examined in mature adipocytes exposed to IL-4, AI, CI or combined treatment in HG (Figure 5A). IL-4 showed no significant effects on MMP-2 (Figure 5A, lane 1). CI stimulated MMP-2 (lane 2) but AI inhibited MMP-2 in HG (lane 3). Intriguingly, CI and AI showed no apparent regulation to MMP-2 under combined treatment (lane 4&5). The above data indicate that while MMP-2 expression in response to IL-4 and AI treatment are similar in pre-adipocytes and mature adipocytes, CI shows opposite regulation to MMP-2 under HG. In addition, the CI regulatory effects are attenuated in mature adipocytes with CI/IL-4 combined treatment.
Figure 5.Effects of insulin and IL-4 on MMP-2 mRNA and activities in mature adipocytes.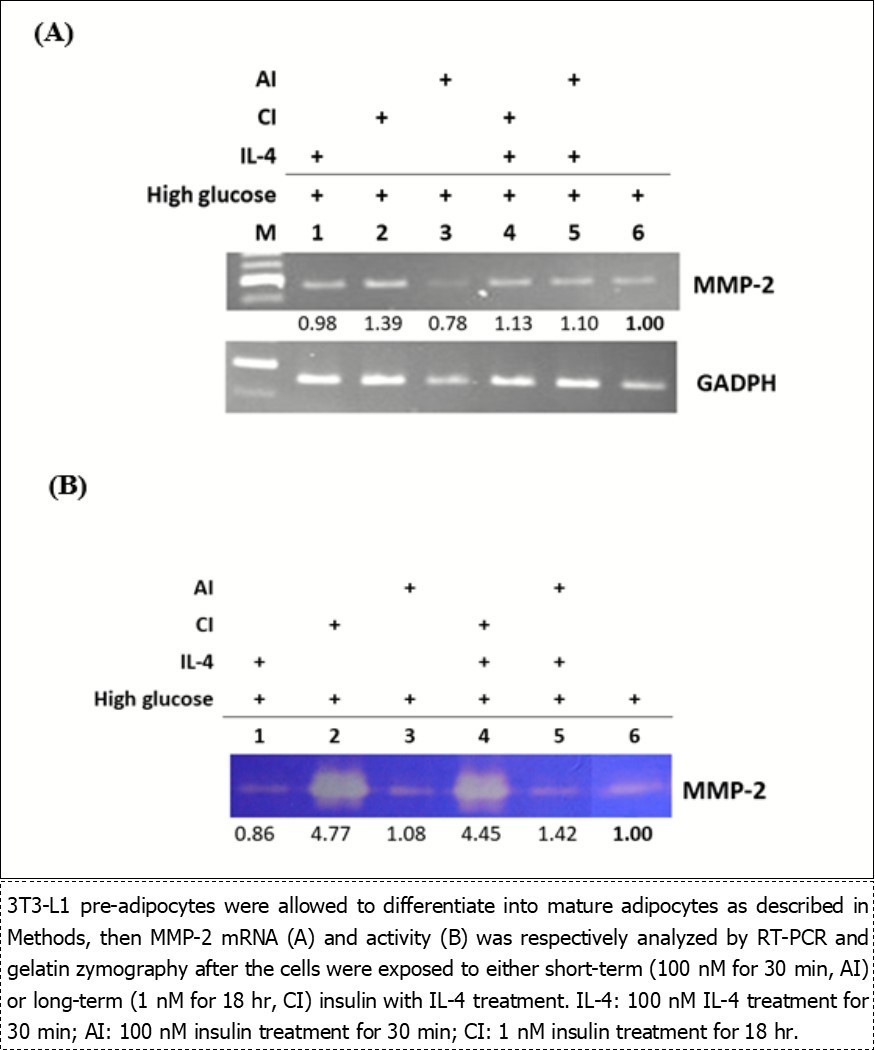
Alterations of MMP-2 activities resulted by IL-4 or insulin treatment in mature adipocytes were subsequently investigated (Figure 5B). Cells were maintained in HG, exposed to IL-4 and/or insulin, then MMP-2 activities were analyzed. IL-4 and AI did not cause prominent alterations of MMP-2 activities (lanes 1&3). In parallel to the results of MMP-2 mRNA (Figure 5A, lane 2), MMP-2 activities were both significantly up-regulated in CI treatment alone (lane 2) and combined treatment (lane 4). Concurrent IL-4 treatment did not cause prominent changes of MMP-2 activity under CI or AI exposure (lanes 4&5). Taken the above observations together, it indicates that CI plays a dominant role by promoting MMP-2 expression and activities in mature adipocytes under HG.
Discussion
Obesity is a pan-endemic health problem closely associated with metabolic disorders and cardiovascular diseases. Most of the obesity-related studies focus on investigating the alteration of adipocytes during the process of increasing adiposity and developing diabetic complications. The corresponding information regarding pre-adipocytes in response to external stimuli under the condition of nutrition homeostasis or oversupply is lacking and remains to be disclosed.
The aims of the present study are to uncover the interaction between IL-4 and insulin to regulate MMP-2 expression and activities in pre-adipocytes under different glucose-containing environments. When cells were maintained in euglycemic media, IL-4 promotes MMP-2 mRNA (Figure 1). Notably, while AI has no effects on MMP-2 activities (Figure 3), CI significantly decreases MMP-2 activities (Figure 4). On the contrary, when cells are in HG media mimicking diabetic hyperglycemia, IL-4 slightly up-regulates MMP-2 (Figure 1) while CI shows reciprocal regulation by suppressing MMP-2 mRNA but enhancing MMP-2 activities (Figure 2 & Figure 3). These results suggest that chronic insulin exposure executes differential MMP-regulating activity in pre-adipocytes. Besides, glucose is a pre-requisite factor for the pre-adipocytes making responses to external stimuli concerning energy metabolism and homeostasis.
It has been shown that MMP-2 is involved in adipogenesis: MMP-2 neutralizing antibodies inhibit adipogenesis6 and MMP inhibitor impairs adipose tissue development.24 In addition, MMP system is implicated in diabetic complications.9,10,11 Glomeruli MMP-2 and the arterial vasculature MMP systemin diabetic subjects are suppressed.25,26 However, contradictory observations exist. Renal MMP-2 protein and activity in diabetic patients are elevated.27,28,29 We also reported elevated MMP-2 expression and activities in T1DM patients.20 Although with conflicting results, the above observations implicate that MMP-2 is critical both for increasing adiposity and the development of diabetic complications. Nevertheless, only very limited information regarding the regulation of MMP-mediated ECM remodeling in pre-adipocytes during obesity-related fat mass development is documented.
Our results reveal that MMP-2 regulatory machinery in pre-adipocytes and adipocytes reacts similarly in response to IL-4 and insulin. Additionally, glucose is a pre-requisite which determines the response of MMP-regulatory machinery in pre-adipocytes (summarized in Figure 6). It indicates that although adipogenesis causes dramatic changes of pre-adipocytes by switching the cells’ characteristics into adipocytes, response of MMP-2 activities remains. Accordingly, in subjects with consistent hyperglycemia, MMP-2 activities and its interaction with the surrounding ECM of pre-adipocytes may react in parallel to that of adipocytes. Therefore, it is tempting to speculate that both mature adipocytes and pre-adipocytes would contribute to ECM dynamics in the pre-diabetic status. Since the MMPs expression abnormality is identified in the lesion of diabetic complications which leads to ECM thickening, our findings also suggest that glucose and insulin levels are the critical factors which participate in the development of diabetic complications by regulating MMP-2 activities in pre-adipocytes. Nevertheless, the above inferences need further evidence.
Figure 6.MMP-2 regulatory machinery in pre-adipocytes and adipocytes reacts similarly towards extracellular stimuli, with glucose concentration as the pre-requisite responding to insulin and/or IL-4 treatment.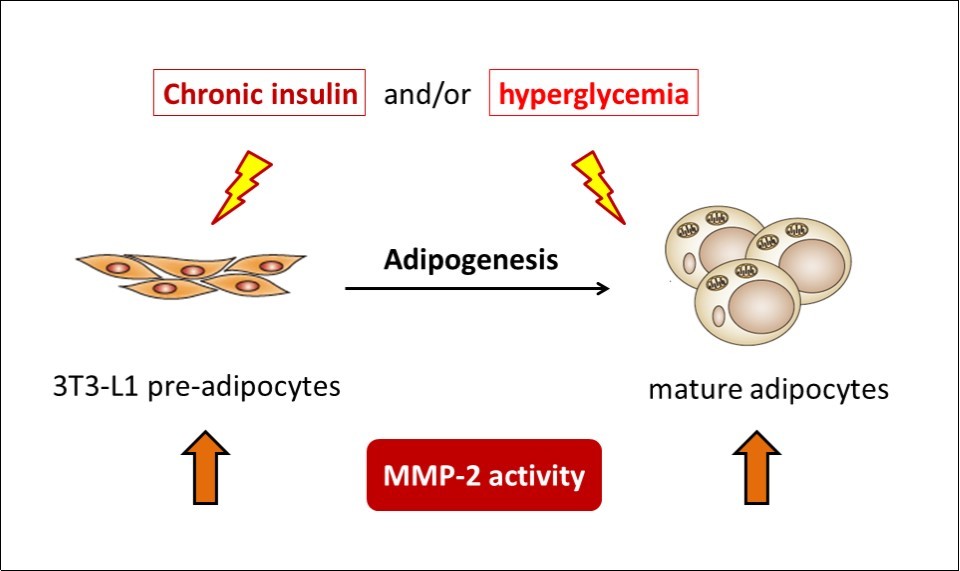
Our results show that chronic insulin exposure has dominant effects on modulating MMP-2 and possibly the ECM remodeling under hyperglycemic state, suggesting pre-adipocytes are likely to participate in the processes of diabetic onset and microenvironmental ECM changes leading to diabetic complications. Although more in vivo investigations have to be performed for verifying the in vitro observations, our data reveal the specific pattern of pre-adipocyte and adipocyte MMP-2 expression under the interaction of environmental glucose, insulin and IL-4. In addition, our findings support the conclusions from several in vivo studies6,30,31,32 that elevated plasma glucose is likely to be pro-inflammatory and thus potentially harmful. On the contrary, insulin administration is likely to be anti-inflammatory and clinically beneficial. Accordingly, patients receiving insulin treatment will benefit from the reduction of the toxic effect elicited by high glucose and the anti-inflammatory effect of insulin. However, further in vivo studies are needed to prove the above speculations due to the limitation of the in vitro evidence provided in the present study.
Conclusion
Our results demonstrate that MMP-2 in pre-adipocytes is regulated by chronic insulin stimulus. This observation suggests that pre-adipocytes may participate in the process of diabetic onset through ECM alterations resulted from MMP-2 changes regulated by glucose or insulin levels way before diabetic onset. Moreover, ECM alterations caused by MMP-2 which are regulated by the interplay between glucose and insulin may contribute to the process of adipogenesis when the individuals are in the process of getting obese, diabetic onset and diabetic complications. The present study uncovers novel observations regarding pre-adipocytes behaviors and their possible roles in the process of increased adiposity. Nevertheless, due to the limitation of in vitro strategies of this study, the speculations regarding the participation of MMP-2 in the process of metabolic abnormalities and complications related to obesity need to be further investigated. We hope this information provides new insights for better understating of metabolic and diabetic pathogenesis and pathophysiology.
Acknowledgements
This work was supported by The Higher Education Sprout Project by the Ministry of Education grant 107AC-D931, and in part by grant MOST 107-2314-B-010-003 from the Ministry of Science and Technology, Taiwan.
List of Abbreviations
AI-Acute insulin treatment
CI-Chronic insulin treatment
ECM-Extracellular matrix
HG-High glucose
IL-4-Interleukin-4
LG-Low glucose
MMPs-Matrix metalloproteinases
MMP-2-Matrix metalloproteinase-2
T2DM-Type 2 diabetes mellitus
References
- 1.Christodoulides C, Lagathu C, J K Sethi, Vidal-Puig A. (2009) Adipogenesis and WNT signalling. , Trends Endocrinol. Metab 20, 16-24.
- 2.H S Camp, Ren D, Leff T. (2002) Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. , Med 8, 442-447.
- 4.Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F. (2000) Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. , In Vitro Cell. Dev. Biol. Anim 36, 38-44.
- 5.Nakajima I, Yamaguchi T, Ozutsumi K, Aso H. (1998) Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. , Differentiation 63, 193-200.
- 6.Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. (2001) Adipocyte produces matrix metalloproteinases 2 and 9 involvement in adipose differentiation. , Diabetes 50, 2080-2086.
- 7.C Mari, Monthouel B, M N Bonnafous, Anglard S, P. (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. , J. Biol. Chem 278, 11888-11896.
- 8.C M Alexander, Selvarajan S, Mudgett J, Werb Z. (2001) Stromelysin-1 regulates adipogenesis during mammary gland involution. , J. Cell Biol 152, 693-703.
- 9.Zaoui P, J F Cantin, Alimardani-Bessette M, Monier F, Halimi S. (2000) Role of metalloproteases and inhibitors in the occurrence and progression of diabetic renal lesions. , Diabetes Metab 26, 25-29.
- 10.H P Marti. (2000) Role of matrix metalloproteinases in the progression of renal lesions. , Presse Med 29, 811-817.
- 11.Diamant M, Hanemaaijer R, J H Verheijen, J W Smit, J K Radder. (2001) Elevated matrix metalloproteinas-2 and -9 in urine, but not in serum, are markers of type 1 diabetic nephropathy. , Diabetes Med 18, 423-424.
- 12.Choi P, Reiser H. (1998) IL-4: role in disease and regulation of production. , Clin. Exp. Immunol 113, 317-319.
- 13.Y H Chang, C N Huang, M Y Shiau. (2012) Association of IL-4 receptor gene polymorphisms with high density lipoprotein cholesterol. , Cytokine 59, 309-312.
- 14.K T Ho, M Y Shiau, Y H Chang, C M, S C Yang. (2010) Association of interleukin-4 promoter polymorphisms in Taiwanese patients with type 2 diabetes mellitus. , Metabolism 59, 1717-1722.
- 15.Y H Chang, K T Ho, S H Lu, C N Huang, M Y Shiau. (2012) Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. , Int. J. Obes. (Lond.) 36, 993-998.
- 16.C H Tsao, M Y Shiau, P H Chuang, Y H Chang, Hwang J. (2014) Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. , J. Lipid Res 55, 385-397.
- 17.M Y Shiau, H F Lu, Y H Chang, Y C Chiu, Y L Shih. (2015) Characterization of proteins regulated by interleukin-4 in 3T3-L1 adipocytes. , SpringerPlus 4, 242.
- 18.M Y Shiau, P S Lee, Y J Huang, C P Yang, C W Hsiao. (2017) Role of PARL-PINK1-Parkin pathway in adipocyte differentiation. , Metabolism 72, 1-17.
- 19.C P Yang, M Y Shiau, Y R Lai, K T Ho, C W Hsiao. (2018) Anti-inflammatory cytokine interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. (in press) , Oxid. Med. Cell. Longev
- 20.M Y Shiau, S T Tsai, K J Tsai, M L Haung, Y T Hsu. (2006) Increased circulatory MMP-2 and MMP-9 levels and activities in patients with type 1 diabetes mellitus. , Mt. Sinai J. Med 73, 1024-1027.
- 21.G De Pergola, Ciccone M, Pannacciulli N, Modugno M, Sciaraffia M. (2000) Lower insulin sensitivity as an independent risk factor for carotid wall thickening in normotensive, non-diabetic, non-smoking normal weight and obese premenopausal women. , Int. J. Obes. Relat. Metab. Disord 24, 825-829.
- 22.Shimobayashi M, Albert V, Woelnerhanssen B, I C Frei, Weissenberger D. (2018) Insulin resistance causes inflammation in adipose tissue. , J. Clin. Invest 128, 1538-1550.
- 23.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. (2009) Insulin as an anti-inflammatory and antiatherogenic modulator. , J. Am. Coll. Cardiol.53(Suppl):S14-20
- 24.H R Lijnen, Maquoi E, L B Hansen, B Van Hoef, Frederix L. (2002) Matrix metalloproteinase inhibition impairs adipose tissue development in mice. , Arterioscler. Thromb. Vasc. Biol 22, 374-379.
- 25.Del Prete D, AnglaniF ForinoM, CeolM FiorettoP. (1997) Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. , Diabetologia 40, 1449-1454.
- 26.Portik-Dobos V, M P Anstadt, Hutchinson J, Bannan M, andErgul A. (2002) Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. , Diabetes 51, 3063-3068.
- 27.A M Romanic, C L Burns-Kurtis, Ao.Z.,ArlethAJ,OhlsteinEH(2001) Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. , Am. J. Physiol, Renal Physiol 281, 309-317.
- 28.T M Camp, S C Tyagi, R M Senior, M R Hayden, S C andTyagi. (2003) Gelatinase B (MMP-9) an apoptotic factor in diabetic transgenic mice. , Diabetologia 46, 1438-1445.
- 29.Noji Y, KajinamiK KawashiriMA, TodoY HoritaT. (2001) Circulating matrix metalloproteinase and their inhibitors in premature coronary atherosclerosis. , Clin. Chem. Lab. Med 39, 380-384.
- 30.Dandona P, Aljada A, Mohanty P, Ghanim H, Bandyopadhyay A. (2003) Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. , Diabetes Care 26, 3310-3314.
