Protective Role of Quercetin Against Rotenone- Induced Hepato and Nephrotoxicity in Swiss Albino Mice
Abstract
Rotenone is well known environmental neurotoxin used to induce Parkinson’s disease (PD) model. Numerous studies are investigated its toxicity on the brain but few studies are available that examined its toxicity on the liver and kidney. Therefore, the main aim of the present work was to explore the toxicity of rotenone on the liver and kidney and its protection through quercetin. Administration of rotenone orally at the dose of (5mg/kg b.w daily for 60 days) caused a significant increase in the levels of liver function and renal function biomarkers as compared to controls. A significant increase in the level of lipid peroxidation, nitric oxide, and decrease in the levels of reduced glutathione, reduction in the activities of catalase and superoxide dismutase were observed in the liver and kidney as compared to control. The histopathological and SEM study in rotenone-treated mice showed alteration and signs of inflammation in the liver and kidney. While co-treatment of quercetin orally (30 mg/kg b.w for 60 days) together with rotenone, reversed the above parameters. In conclusion, rotenone significantly damages the liver and kidney, and the administration of quercetin along with rotenone shown a protective role. This study provides a new insight into where rotenone-induced liver and kidney dysfunction could be successfully protected by quercetin.
Author Contributions
Academic Editor: Abdelmonem Awad Mustafa Hegazy, Professor and Former Chairman of Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Juli Jain, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Most of the human health problems and environmental pollutions are caused by the use of pesticides to improve crop production and pest control in agriculture 1. despite their fame and prevalent use, pesticides have to indicate serious warnings towards the health risks of farmers who mix and use pesticides or work in treated areas 2. Several pesticides such as paraquat, maneb, dieldrin, and rotenone are a possible hazard to humans due to their documented neurotoxicity in animals. 3. The study has been also reported that depression and neurotoxicity were caused in agriculture workers exposed to pesticides 4.
Rotenone is an organic pesticide extracted from Leguminosae plants. Now rotenone known as an environmental toxin causes neurological disorders like PD 5. Mechanism of action involves rotenone inhibits the mitochondrial complex I, as a result, reactive oxygen species (ROS) are produced which leads to oxidative stress, reducing ATP production 6, lowering the membrane potential of mitochondria 7, and finally causes cellular death 8. Oxidative stress produced by rotenone has been strongly implicated in the pathophysiology of PD 9. ROS generation is a natural physiological process in different organs. But, excessive generation of ROS can be damaging to the hepatic system 10 and nephritic system 11. The usual function the the brain is intimately associated with the normal function of the liver and kidney. Dysfunction of the liver and kidney plays a critical role in the development of neuro disorders and cognitive impairment 12, 13. Liver function abnormality leads to deficient detoxification thus permitting neurotoxins like manganese, ammonia, and other chemicals to pass in the cerebral circulation. Chronic kidney disease (CKD) is related to a high prevalence of cerebrovascular disorders like stroke, intracerebral microbleeds, and cognitive impairment. This condition has been found not only in patients with end-stage renal disease but also in patients with slight or reasonable CKD 14. Recently a finding suggests that liver dysfunction from mild steatohepatitis to cirrhosis and hepatic encephalopathy leads to, microglial activation and loss of Purkinje neurons in the cerebellum of patients with Steatohepatitis 15. Cognitive alterations and dementia were found in patients with chronic kidney disease 16. Increased oxidative stress in the liver of mice was found when treated with rotenone 17. Rotenone administration caused oxidative damage in renal tissue, causing overproduction of malonaldehyde and ROS, reduction in glutathione, blocking SOD, and glutathione peroxidase activity in rats.
Flavonoids as strong free radical scavengers have fascinated the remarkable interest of researchers 18, 19 as possible therapeutics, against diseases induced by free radical 20. Quercetin (3,5,7,3’,4 pentahydroxyflavone), plant-derived flavonoids found in vegetables and fruits. They have been documented to possess several pharmacological activities involving anti-inflammatory, antiallergic, and antioxidant properties 19, 21. Quercetin can directly scavenge the superoxide anion and blocks numerous superoxide-producing enzymes like xanthine oxidase 22, 23, 24 or the neutrophil membrane NADPH oxidase complex 25. Among the other antioxidant like Vitamin E and Vitamin C, quercetin is stronger than these 26, 27, 28. Therefore, the main aim of the present study is to investigate the potential protective effects of quercetin toward histopathological and biochemical changes induced by rotenone in the liver and kidney. This study also helps in prevent subsequent neuro disorders due to compromised function of the liver and kidney. Our finding contributing to a novel approach in the treatment of neurological disorders caused by liver and kidney dysfunction through quercetin.
Materials and Methods
Chemicals
Hydrochloric acid (HCl), ethanol, sodium hydroxide (NaOH), Trichloroacetic acid (TCA), 5,5’-dithiobis 2-nitrobenzoic acid (DTNB), di-nitrophenyl-hydrazine (DNPH), Bovine serum albumin, Folin- ciaculate, paraffin wax, and quercetin were purchased from HiMedia Laboratories (Mumbai, India). Potassium dihydrogen orthophosphate (KH2PO4), sodium chloride, and ethylene diamine tetraacetate (EDTA) were purchased from LOBA, Chemie, India. Rotenone was purchased from MP Biomedicals, France.
Experimental Animals and Treatment
Adult Swiss albino mice, 3-4 months old weighing (25-30 g) were purchased from the College of Veterinary Science and Animal Husbandry Mhow, India, and acclimatized for 2 weeks before starting the experiment. Mice were housed in polypropylene cages under standard hygiene conditions, at temperature between 24-28°C with a 12-hour light/dark cycle, and were given standard food and water ad libitum.
The mice were randomly divided into three groups having 6 mice in each group. In group I, mice were treated with vehicle and served as the control, in group II, mice were treated with rotenone (5 mg/kg dissolved in sunflower oil) for 60 days, group III were co-treated with quercetin (30 mg/kg dissolved in sunflower oil) and rotenone for 60 days.
For the biochemical study, after the last day of treatment, four mice from all the experimental groups were anesthetized under ether anesthesia and blood was collected by cardiac puncture. Blood was used for serum preparation and the liver and kidney were excised rapidly and washed with a chilled saline solution. Tissue was weighed and homogenized in homogenate buffer phosphate buffer 0.1 M PH 7.4 (10% w/v) using Teflon homogenizer (Remi Stirrer, 125S/U, Bombay India). Homogenate was centrifuged (1000 rpm for 10 min at 4 °C) to remove the debris and the supernatant was taken out. The supernatant was again centrifuged (12,500 rpm for 15 min at 4 °C), and the pellet was discarded. The clear supernatant fluid was taken for analysis of Lipid peroxidation (LPO), Nitric Oxide (NO), Superoxide dismutase (SOD), Catalase, and Glutathione (GSH) assays. All procedures were done in accordance with Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and the study was approved by the Institutional Animal Ethical Committee (IAEC No. 379/GO/ReBi/S/01/CPCSEA), Dr. Harisingh Gour University, Sagar (MP), India.
Markers of Liver and Kidney Functions
Blood was collected by cardiac puncture into, serum gel separator tubes (Thyocare) and centrifuged at 3000 rpm for 10 min and serum was used for the assessment of liver function markers and kidney function markers. Activities of serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and the level of urea and creatinine were measured by the Thyocare laboratory Sagar (M.P).
Biochemical Analysis
Determination of LPO: The level of Lipid peroxidation (LPO) measured by the amounts of MDA present in the homogenate of tissue by the use of the thiobarbituric acid (TBA) colour reaction at 95°C temperature for 40 minutes to produce a TBA-reactive substance and then resultant pink-colored pigment trimethylene complex product was measured maximum absorbance at 532 nm using spectrophotometer by the method of 29. The results were expressed as nmol MDA/mg protein.
Determination of GSH: The content of Reduced glutathione (GSH) was estimated by the procedure of 30. DTNB (5,5’-dithiobis (2-nitrobenzoic acid) or Ellman´s reagent is reduced by the free sulfhydryl group on GSH molecule to yield generate 5-to-2-nitrobenzoic acid which has a yellow color and can be determined by reading absorbance at 412 nm and expressed as umol/mg protein.
Determination of NO: Nitric Oxide (NO) is produced from arginine by nitric oxide synthase (NOS). Nitrate is converted to nitrite by nitrate reductase. The level of nitric oxide was determined by measuring the accumulation of nitrite using Greiss reagent. Nitrite ions react with Griess reagent to form a pink diazo dye by diazonium coupling reaction which is measured at 540 nm as described earlier by 31 and expressed as μMol/mg tissue.
Determination of SOD: Superoxide dismutase (SOD) activity was determined spectrophotometrically at 560 nm using the modified method of Kakkar 32. Briefly, the assay mixture containing sodium pyrophosphate buffer (0.052M, pH 8.3), nitroblue tetrazolium (300 uM), phenazine methosulfate (186 uM), NADH (780 uM), and appropriately diluted enzyme in the total volume of 3mL was incubated at 37°C for 90 s. The reaction was stopped by the addition of glacial acetic acid. The reaction mixture was mixed vigorously by adding n-butanol and was allowed to stand for 10 min before the collection of the butanol layer. The intensity of the chromogen in butanol was measured at 560 nm. The SOD activity was calculated in the inhibition rate %.
Determination of Catalase Activity: Catalase was measured by the method as described by 33, spectrophotometrically in a post-mitochondrial fraction using H2O2 as a substrate. The activity of the catalase was expressed in mmole/min/mg protein.
Protein Estimation: Protein concentration was estimated by 34 using BSA as a standard.
Histopathological Study
After the last day of treatment, two mice from each treatment group were perfused transcardially with ice-cold 0.1 M phosphate-buffered saline (PBS) followed by cold paraformaldehyde (4%, w/v) in 0.1 M PBS. After that, the liver and kidney were isolated and processed for paraffin-embedded sectioning. Six-micron sections were cut with a microtome and mounted on clean gelatin-coated slides, and stained by hematoxylin and eosin, and observed under a light microscope.
Scanning Electron Microscopy (SEM)
SEM study of liver and kidney sample was performed on paraffin sections organized for histology. 6 um thick sections of liver and kidney were dried at 37°C for overnight and deparaffinized in xylene for 20 min. After that, sections were rinsed with trichloro-trifluoro-ethane (C2Cl3F3) and the solvent was seared by evaporation. Then, the backscattered electrons (BSE) were used for noticing the various constituents in the liver and kidney from the surface of seared sections. The surface conductive coating of gold (Au) was done for further visualization. Images were taken at different magnifications i.e. low and high 35 through scanning electron microscopy, FEI Nova NanoSEM 450 made in Netherland.
Statistical Analysis: The statistical significance was determined by one-way analysis of variance (ANOVA) by the use of Sigmaplot version 12.0. Values were expressed as mean ± standard error of the mean (SEM). The level of significance was set at p <0.05.
Results
Effect on Body Weight
The results of body weight of mice from rotenone treated mice showed that a significant decreased at all different days from day 16 th to 32 th(p <0.01) and 40 th to 56 th (p <0.001) as compared to control Figure 1. Co- administration of quercetin leads to the increased body weight at 40 th (p <0.01) , 48 th (p <0.001) and at 56 th(p <0.05) as compared to rotenone alone group Figure 1.
Figure 1.Effect of rotenone and co-treatment of rotenone and quercetin on body weight of mice. The results were expressed as mean±SE (n=06). ***p<0.001), **(p<0.01) Significantly differs from control group, ###(p<0.001), ##(p < 0.01), #(p < 0.05) Significantly differs from rotenone treated group.
Effect on the Liver Function Markers
The results of the present study showed that administration of rotenone caused significant increases in levels of liver-function biomarkers, i.e., AST (3.28 folds, P < 0.001), ALT (3.03 folds, p < 0.001), and ALP (1.81 folds, p < 0.001), compared to the control (Table 1). While co-treatment with quercetin along with rotenone decreased the liver-function biomarkers, i.e., AST (1.30 folds, p < 0.05), ALT (1.18 folds, p < 0.05), and ALP (1.32 folds, p < 0.01), compared to the rotenone alone.
Effect on the kidney function markers Levels of biomarkers related to kidney functions, i.e., increased significantly urea (1.64 folds, p < 0.05) (Table 1), and creatinine (3.25 folds, p < 0.001) (Table 2) then these of the control. Levels of biomarkers of kidney functions, i.e., decreased significantly when co-treated with quercetin urea (1.24 folds, P < 0.05) (Table 2), and creatinine (1.66 folds, p < 0.001) (Table 2), compared to the rotenone alone group.
Table 1. Effect of rotenone and co-treatment of rotenone and quercetin in the activities of serum hepatic markers in mice. ***(p < 0.001), Significantly differs from control group, ###(p < 0.001), #(p < 0.05) Significantly differs from rotenone treated group.| Parameters | Control | Rotenone | Rotenone + Quercetin |
| Alkaline phosphatase (U/ml) | 62.32±6.16 | 113.17±3.32*** | 85.4±2.32### |
| Aspartate aminotransferase (U/ml) | 30.43±3.14 | 99.83±4.61*** | 76.60 ±3.98# |
| Alanine aminotrans-ferase (U/ml) | 27.73± 2.25 | 84.06±2.69*** | 71.07± 2.30# |
| Parameters | Control | Rotenone | Rotenone + Quercetin |
| Urea mg/dl | 18.94±2.33 | 31.07±2.13* | 25.04±1.54# |
| Creatinine mg/ dl | 0.16±0.02 | 0.43±0.02*** | 0.26±0.01### |
Effect on LPO
The results of LPO revealed that the LPO level was significantly increased in the liver (2.0 folds, p <0.001) and kidney (1.47 folds, p <0.001) in rotenone-treated mice as compared to control Figure 2. Co-treatment with quercetin along with rotenone significantly decreases the LPO level in the liver (1.84 folds, p <0.001) and kidney (1.79 folds, p <0.001) as compared to those treated with rotenone alone.
Figure 2.Effect of rotenone and co-treatment of rotenone and quercetin on lipid peroxidation in liver and kidney of mice. The results were expressed as mean±SE (n=04). ***p<0.001) Significantly differs from control group, ###(p<0.001) Significantly differs from rotenone treated group.
Effect on NO Levels
NO level was increased significantly (2.34 folds, p <0.001) in the liver and in the kidney (1.79 folds, p <0.001) in the rotenone treated group as compared to control Figure 3. Co-treatment with quercetin along with rotenone decreased the level of NO in the liver (2.41 folds, p <0.001) and in the kidney (1.95 folds, p <0.001) as compared to those treated with rotenone alone.
Figure 3.Effect of rotenone and co-treatment of rotenone and quercetin on nitric oxide levels in liver and kidney of mice. The results were expressed as mean±SE (n=04). ***p<0.001) Significantly differs from control group, ###(p<0.05) Significantly differs from rotenone treated group.
Effect on GSH
GSH content was significantly decreased (1.22 folds, p <0.01), (3.15 folds, p <0.001) respectively in the liver, kidney of rotenone treated group as compared to control Figure 4. Co-treatment with quercetin along with rotenone significantly increased the level of GSH (1.45 folds, p <0.001), (1.67 folds, p <0.01) respectively as compared to those treated with rotenone alone.
Figure 4.Effect of rotenone and co-treatment of rotenone and quercetin on reduced glutathione in liver and kidney of mice. The results were expressed as mean±SE (n=04). **(p<0.01) Significantly differs from control group, (##p<0.01, ###p<0.001) Significantly differs from rotenone treated group.
Effect on SOD Activity
The activity of SOD was significantly decreased (1.34 folds p <0.001), (1.24 folds, p <0.001) respectively in the liver, kidney of rotenone treated group as compared to control Figure 5. Co-treatment with quercetin along with rotenone significantly increased the activity of SOD (1.15 folds, p <0.05),(1.12 folds, p<0.001) in the liver and kidney respectively as compared to those treated with rotenone alone.
Figure 5.Effect of rotenone and co-treatment of rotenone and quercetin on catalase in liver and kidney of mice. The results were expressed as mean±SE (n=04). ***(p<0.001), Significantly differs from control group, ###(p<0.001) Significantly differs from rotenone treated group.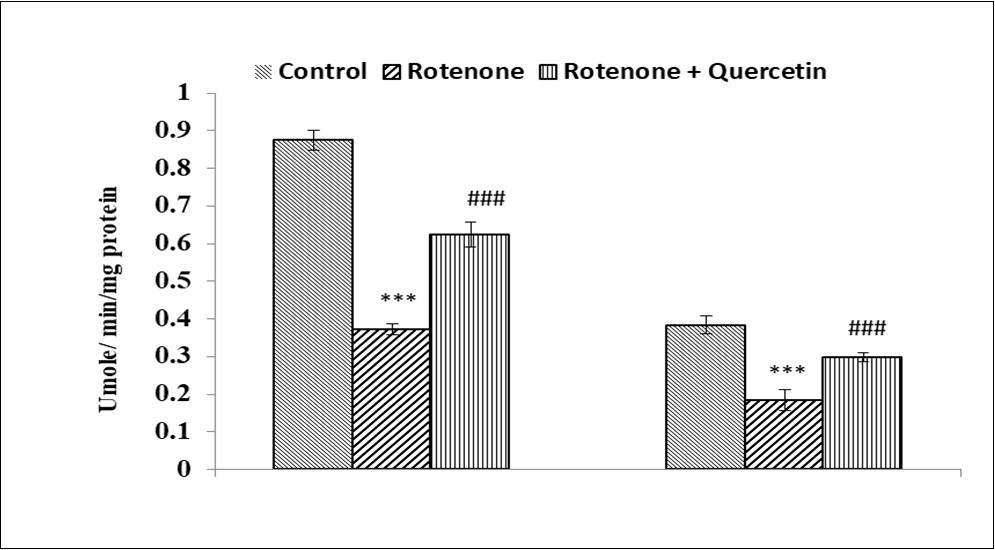
Effect on Catalase Activity
The activity of catalase was significantly decreased (2.02 folds, p <0.001), (2.07 folds, p <0.001) respectively in the liver, kidney of rotenone treated group as compared to control Figure 6. Co-treatment with quercetin along with rotenone significantly increased the activity of catalase (1.67 folds, p <0.001), (1.60 folds, p<0.001), in liver and kidney respectively as compared to those treated with rotenone alone.
Figure 6.Effect of rotenone and co-treatment of rotenone and quercetin on superoxide dismutase in liver and kidney of mice. The results were expressed as mean±SE (n=04). ***(p < 0.001) Significantly differs from control group, #(p<0.05) Significantly differs from rotenone treated group.
Effect on Histological changes in Liver and Kidney
The histological investigation of liver sections from the control mice Figure 7A (A) showing no histopathological alteration indicating normal architecture of hepatocytes cells (green asterisks), intectshepatic strands (yellow arrows), Blood sinusoid (Bs, red line), and central vein (cv). Rotenone treated group, showing degranulation of hepatocytes cytoplasm, loss of hepatic strands (yellow arrows), abnormality in sinusoids (red line), shrinkage of hepatocytes (green asterisks), leucocytes infiltration (green arrow head), and Kupper cells activation (black arrowhead). When co-treated with quercetin along with rotenone showing classical hepatic strands, the normal structure of blood sinusoids (Bs), central vein (cv), and the normal appearance of hepatocytes (green asterisks) and least no of Kupper cells activation. Inflammatory cellular infiltration was found more around the central vein in the rotenone treated group. The results of present studies suggested that quercetin protects against liver injury due to rotenone-induced inflammation and histological alteration.
Figure 7A.Effect of rotenone and co-treatment of quercetin and rotenone on histology of liver of mice. Control liver showing normal appearance classic hepatic strands (yellow arrows) with hepatocytes (green asterisk) of control mice separated by blood sinusoids (Bs) (red line); Central vein (CV). Rotenone treated group, showing degranulation of hepatocytes cytoplasm, loss of hepatic strands (yellow arrows); abnormality in sinusoids (red line); shrinkage of hepatocytes ( green asterisks),Leucocytes infiltration (green arrow head) and Kupper cells activation (black arrow head). Rotenone + quercetin treated group showing classical hepatic strands (yellow arrows); normal structure of blood sinusoids (Bs); central vein (Cv); normal appearance of hepatocytes ( green asterisks) and least number of Kupper cells activation (black arrow head). Tissues sections (about 5 μm) were prepared, stained with haematoxylin and eosin (H&E). Magnification, 40×
Figure 7B.Scanning electron micrographs of liver of rotenone and co- administration of quercetin mice . Control group showing normal appearance of hepatocytes strands ( red arrow), central vein (yellow star); surrounding the normal sinusoids (yellow line ), Rotenone administrated group, shows loss of hepatic strands (red arrows); abnormality in sinusoids (Yellow line). Rotenone + quercetin treated group showing classical hepatic strands ,normal structure of blood sinusoids , central vein and normal appearance of hepatocytes .Scale Bar 200 μm and 10 μm.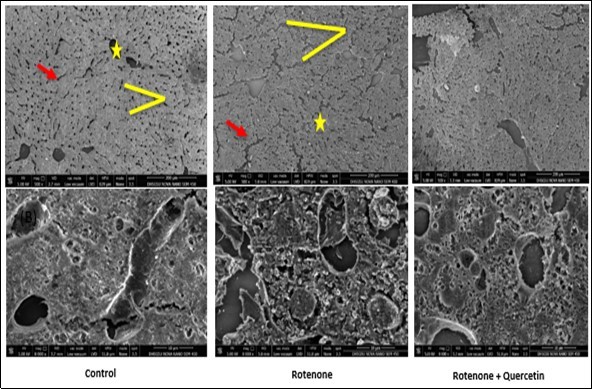
The histopathological investigation of the kidney of control mice showed normal architecture of renal corpuscles with their glomeruli and renal tubules. Rotenone treated mice showing alteration in renal corpuscles, diffuse hydropic degeneration of renal tubular epithelium, multifocal chronic interstitial nephritis, considered by lymphocyte infiltration, mononuclear cells in interstitium and necrosis Figure 8A. While mice treated with quercetin along with rotenone showing a normal appearance of renal corpuscles and renal tubules.
Figure 8A.Effect of quercetin and co-treatment of quercetin and rotenone on histological alteration of mice kidney following exposure to rotenone.(A) Control group showing normal architecture of renal corpuscles with their glomeruli (Yellow arrows) and renal tubules (green arrow head). Photomicrograph of kidney treated with rotenone showing alteration in renal corpuscles (yellow arrows); degeneration of renal tubules (green arrow head) and necrosis and wide spacing of tubules with atrophy of their lining epithelium (red star), focal interstitial chronic inflammation infiltration of lymphocytes and mononuclear cell interstitium (blue arrow). Photomicrograph of kidney treated with rotenone + quercetin showing normal appearance of renal corpuscles and renal tubules. Kidney tissue sections were stained with hematoxylin and eosin method (40x).
Figure 8B.Scanning electron micrographs of kidney of rotenone and co- administration of quercetin mice . Control group showing normal architecture of renal corpuscles with their glomeruli (Yellow arrows) and renal tubules (green arrow head). Photomicrograph of kidney treated with rotenone showing alteration in renal corpuscles (yellow arrows); degeneration of renal tubules (green arrow head), wide spacing of tubules with atrophy of their lining epithelium (red star).Photomicrograph of kidney treated with rotenone + quercetin showing normal appearance of renal corpuscles and renal tubules.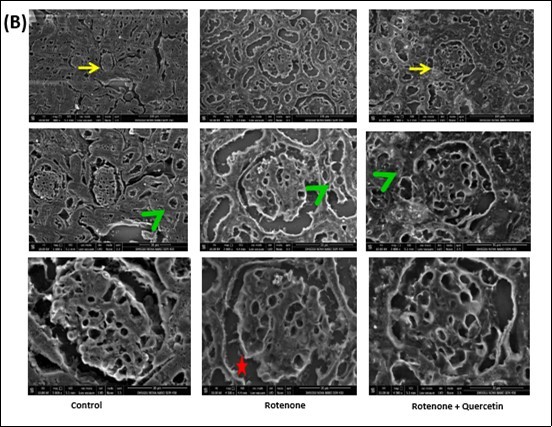
Scanning Electron Microscopy of Liver and Kidney
Scanning electron microscopy (SEM) has enhanced our understanding of the complex morphology of the liver and kidney. Investigation of the liver tissue at low magnification resulted in the inclusive remark of the histological construction of the functional liver unit. In SEM analysis of control mice, the hepatocytes strands are seen in the normal manner. As shown by the photomicrograph, a row of hepatocytes lies between two sinusoids, (Figure 7B). Rotenone administrated mice revealed broken hepatocytes strand and irregular shape of central vein and blood sinusoids as compared to control group. Co-administration of quercetin effectively restores the morphology of liver hepatocytes cells with the intact structure of hepatocytes strands and blood sinusoids (Figure 7B).
Scanning Electron Microscopy of Kidney
Scanning electron microscopy (SEM) has suggested about complex morphology of kidneys of mice. SEM investigation of control mice shown the typical structure of renal corpuscle and renal tubules (Figure 8B). Rotenone administrated mice displayed irregularities in renal corpuscle and renal tubules with widespread spacing in epithelium cells compared with control mice. Co-administration of quercetin fruitfully avert the morphology of the kidney (Figure 8B).
Discussion
The present work investigated the ameliorative role of quercetin against rotenone-induced alteration in the liver and kidney of mice at various biochemical parameters and histological levels. Treatment of rotenone for 60 days leads to the increased LPO, NO also known as the inflammatory marker, and a noticeable reduction in the content of GSH, activities of catalase, SOD, and histological changes in liver and kidney were observed. The liver and kidney showed signs of increased oxidative stress. Taken together, Co-administration of quercetin decreased oxidative stress and ameliorate rotenone toxicity.
Evidence from the earlier study suggested that exposure to pesticides altered the antioxidant defense system in the brain, heart, and kidney of rats by producing oxidative stress, leading to esevere damage 36, 37, 38. Rotenone a well-known environmental toxin induces oxidative stress by inhibiting complex I of the electron transport chain. Recently, in our lab, we reported that exposure to rotenone was confirmed to caused oxidative stress in both in vitro as well as in vivo mice models 17. Various studies have observed that quercetin, a natural antioxidant has strong free radical scavenging activity and sthe could protect against oxidative damage 39.
Measurment of body weight is the key indicator of development and physical growth of the organisms. In PD gastrointestinal dysfunction is the main characteristics during rotenone administration with other symptoms such as weight loss, constipation and early satiety 40 Treatment of rotenone could be linked with late in gastric emptying which is the cause of reduced body weight of animals41. Rotenone administrated rats have been shown reduced body weight continuously during admistration of rotenone and significant decreased in body weight were found at the end of 4th week42. Supporting these finding in our present work, we observed a significant reduction in body weight in rotenone administrated mice. These outcome significantly reversed by co-treatment with quercetin along with rotenone.Our study consistence with other published data in which quercetin has been found to Prevents body weight loss against toxicity of superparamagnetic iron oxide nanoparticles in rats 43
The liver is the primary target of ingested oxidants and also the main tissue involves in the protection against oxidative stress. Liver dysfunction is associated with increased levels of serum enzymes, which are the suggestive sign of cellular leakage and loss of functional integrity of cellular membrane in the liver 44. High levels of AST and ALT are well parameters to identify liver damage 45. Serum GGT and ALP levels are also related to the status and function of hepatic cells. In the present study elevated levels of hepatic markers like ALP, ALT, and AST in the serum proposed that an extensive liver injury was caused by rotenone. Indicating rotenone causes structural and functional damage to the cell membrane and enhanced membrane permeability leading to the leakage of hepatic enzymes into the blood. It is well established that rotenone administration significantly increased the serum hepatic marker enzyme 46. Co-administration of quercetin decreased the above liver function biomarkers in blood serum. Our result has been supported by another study, quercetin reduced the liver function biomarkers from acute liver injury in rats induced by composite factors 47. Administration of quercetin effectively decreased the activities of these enzymes in the present study. This can be attributed to the antioxidant property of quercetin and membrane-stabilizing property.
The kidney is the vital organ of the body it plays a major role in homeostasis and regulates the extracellular environment that helps in the excretion and detoxification of drugs and metabolites, which are harmful to the body48. For this reason, it is important for exogenous toxicants. By the negative impact of chemicals or drugs, excretions get altered which leads to nephrotoxicity 49. When the function of the kidney is altered and the homeostatic function disturbed it is unable to get rid of body excess waste50, 51. In a study, it has been reported that treatment of rotenone caused the increased serum urea and creatinine and MDA level, protein carbonyl content, and reduction in GSH level, and activities of, GST, SOD, MPO, and LDH have been observed 52. Rotenone toxicity also caused an enhanced level of serum urea and creatinine, which are central metabolites associated with renal health 53. At the time of skeletal muscle metabolism creatinine, is formed by the spontaneous and irreversible reaction. Increased creatinine level in kidneys connected with renal damage 54. One of the major incidents in nephrotoxicity is an increase in the level of serum urea due to alteration in kidney function55.
Research has been found that treatment of rotenone induces ROS production and structural damage in renal proximal tubule derived from OK cells 11. Another study also reported that rotenone administration leads to oxidative stress, causingan increased level of malonaldehyde, and ROS, and blocking SOD and glutathione peroxidase (GSH-Px) activity. Along with this due to rotenone treatment reduction in the membrane potential of mitochondrial and increased voltage-dependent anion channel (VDAC), caspase-3, and caspase-9 protein levels in the kidney were found [56]. In the present study increased levels of urea and creatinine were observed in rotenone administrated mice signifying severe renal damage. The enhanced degree of these kidneys functions markers in serum linked to oxidative stress and inflammation from rotenone-induced damaged [53, 57]. The serum biochemistry results obtained from this study also showed an increased level of urea and creatinine suggesting severe renal damage occurred in mice treated with rotenone. Our result supported by another study increased levels of urea and creatine were observed in rotenone-intoxicated rats [58]. Co-administration of quercetin decreased the level of urea and creatine. Consistent with our work quercetin reduces serum creatinine and urea nitrogen levels against the toxicity of a mixture of four organophosphate pesticides [59].
Excessive generation of ROS encourages oxidative stress following pesticide exposure, leads to lipid peroxidation 60. In view of the present results, mice administered rotenone displayed a significant enhancement of MDA content in the liver and kidney when compared with the control group. These results are in agreement with the earlier findings that reported enhance levels of MDA in the liver and kidney of mice exposed to rotenone 10. There was an enhanced level of the LPO, indicating an attack on membrane lipids by intracellular ROS metabolites 61. Promotion at the LPO level may be one of the primary mechanisms due to pesticide exposure 60.LPO has participated in numerous unfavorable effects in cells like enhanced membrane rigidity and osmotic fragility 62. Co-administration of quercetin protects against rotenone-induced LPO. The free radical scavenging effects of quercetin may be linked with the presence of two hydroxyl groups in the β-ring of its molecule 63. Quercetin posses antiperoxidative properties due to the presence of polyunsaturated substitution on the β-ring together with 2,3 double bond, a free 3-hydroxyl substitution, and a 4-keto group in its structure 64. Several studies reported that LPO was blocked by quercetin administration 65. Hence, in our study co-administration of quercetin effectively scavenges free radicals, blocks LPO, and protects the liver and kidney tissue from the rotenone-induced oxidative damage (Figure 2).
NO is a signaling molecule, which controls several physiological and pathophysiological processes in the immune system, nervous system, and liver 66, 67. It was documented that rotenone increases the content of NO 68. It was also reported that rotenone treatment increases the NO level in the liver and kidney 17, 69. Consistent with this study in the present study we also found increased NO content in liver and kidney increased level of NO also shown the sign of inflammation in liver and kidney induced by rotenone. Co-administration of quercetin decreased the NO content in the liver and kidney (Figure 3). In our previous study, we also observed that the level of NO enhanced in the cerebellum of mice when treated with rotenone and reduced significantly when co-treated with quercetin 70.
GSH is a non-enzymatic member of the antioxidant defense system and serves as a free radical scavenger, but it also participated in liver and kidney protection against various toxicity as a substrate for antioxidant enzymes GSH-Px and GST 71. Our finding has shown that reduction in GSH level in the liver and kidney of rotenone-treated mice are linked with its primary role in oxidative stress condition. Reduction in GSH may enhance the susceptibility of the liver and kidney to pesticides toxicity 72. The administration of rotenone reduced GSH content in the liver which is in accordance with other published studies 17. Co-administration of quercetin restores the GSH content as compared to the rotenone alone group. Quercetin enhances GSH dependent protection and prevents the depletion of thiols during oxidative stress 73. In this study, co-administration of quercetin exerted an antioxidative effect, reversing the alteration in liver and kidney levels of LPO and GSH these effects might have been contributed to the observed different organ protection. The antioxidant role of quercetin may include the inhibition of free radicle, 46 Scavenging of O2-, OH-, peroxyl radicals, and peroxynitrite 74.
SOD is known to be the first line of defense against the harmful effects of free radicals and it scavenges ROS by catalyzing the dismutation of superoxide to H2O2. Studies have shown that rotenone significantly decreased SOD activities in the liver 46. The inhibition of SOD activity may be the consequence of increased flux of superoxide in cells which may be the reason for the enhanced lipid peroxidative indices in our present work. Catalase serves as a preventive antioxidant and plays a major role in the prevention of the harmful effects of LPO. In the present study, there is a significant decrease in the activities of catalase and SOD in the liver and kidney of rotenone-treated mice were found. While co-treatment of quercetin enhanced the activity of catalase and SOD in the liver and kidney. Our study has an agreement with another study where oxidative stress-induced toxicity, by doxorubicin and cyclophosphamide in rat kidney and liver protected by quercetin 75.
The toxicity of rotenone was also confirmed by histological studies. Evidence from the study suggested that histological alteration in rat liver was observed in the rotenone exposed group 69. In this study, histological analysis revealed that rotenone induces striking pathological alteration in the liver, and signs of inflammatory response were observed as indicating by Kupper cell activation and leucocytes infiltration as compared to the control group. Co-treatment with quercetin leads to appear normal architecture of hepatocytes cell and reduction in Kupper cell activation were observed. Quercetin was found to reversed histological alteration induced by a high-fat diet in mice 76. Exertion of metabolite products occurs through the renal tubular epithelium, which might lead to xenobiotic-induced cell damage and hydropic degeneration of tubular epithelium and also able to induce inflammatory cytokine to excite lymphocytes and mononuclear cells infiltrated lesion. In the kidney, rotenone showed severe damage in renal corpuscles, renal tubules as compared to control while co-treatment with quercetin restore the normal appearance of renal corpuscles and renal tubules in the kidney. Protective effects of quercetin on histological changes in kidneys were observed, toxicity induced by methotrexate in rats 77. Scanning electron microscopic study further strongly supported our histological result of liver and kidney where we found severe damage and liver and kidney and protective effects of quercetin. These results suggest that rotenone is hepatotoxic and nephrotoxic and co-administration of quercetin successfully protected liver and kidney damage caused by rotenone.
Conclusion
In conclusion, the outcome of the present study showed that rotenone caused alteration in liver and kidney function biomarkers together with this also altered the biochemical parameters and induced inflammatory response as indicated by an increased level of No and presence of inflammatory cells. Medicinal plants with strong pharmacological properties have been found to play a key role in the health care system of huge amounts of the world’s populations. Comprehensive research on the chemistry and pharmacology of components of plant origin are essential and this may ultimately lead to the finding of medicine that can be used in dealing with numerous diseases. Biological products with antioxidant, anti-inflammatory activities allow the prevention of cells and tissues against harmful effects of free radicals by averting or governing the procedure of harm. In our study co-administration of quercetin leads to a reduction in damaged caused by rotenone. The possible mechanism of quercetin as a hepatoprotective and nephroprotective may be due to its anti-oxidant and anti-inflammatory. These results also suggest that toxicity of rotenone not only limited to the brain as reported by other literature but also affect the other organ which further contributes in neurological disorders due to liver and kidney dysfunction as suggested by various published data. Quercetin significantly protected the liver and kidney dysfunction, suggesting that quercetin can protect liver and kidney damage, maybe it further inhibit neurological disorders associated with liver and kidney dysfunction. This study might be helpful to the pharmacologist in the development of the drug in the treatment of PD through targeting the liver and kidney also. Further investigations are required to find out molecular mechanisms of quercetin and their action against rotenone or other pesticides with adverse effects on non-target organisms.
Acknowledgments
We thank Dr. Harisingh Gour University, Sagar (M.P) India for their support.
Funding
The authors didn’t receive any financial support for the research work, authorship and publication of this article.
References
- 1.Soares W, Almeida R M, Moro S. (2003) [Rural work and risk factors associated with pesticide use in Minas Gerais, Brazil]. Cadernos de saude publica 19:. 1117-27.
- 2.Soares W L, MF de Souza Porto. (2009) Estimating the social cost of pesticide use: An assessment from acute poisoning in Brazil. , Ecological Economics 68, 2721-8.
- 3.Liu B, Gao H M, Hong J S. (2003) Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environmental health perspectives 111:. 1065-73.
- 4.Kori R K, Mandrah K, Hasan W, Patel D K, Roy S K et al. (2020) Identification of markers of depression and neurotoxicity in pesticide exposed agriculture workers. Journal of biochemical and molecular toxicology 22477.
- 5.Du C, Jin M, Hong Y, Li Q, Wang X H et al. (2014) Downregulation of cystathionine beta-synthase/hydrogen sulfide contributes to rotenone-induced microglia polarization toward M1 type. Biochemical and biophysical research communications 451:. 239-45.
- 6.Madiha S, Haider S. (2019) Curcumin restores rotenone induced depressive-like symptoms in animal model of neurotoxicity: assessment by social interaction test and sucrose preference test. , Metab Brain Dis 34, 297-308.
- 7.Tamilselvam K, Braidy N, Manivasagam T, Essa M M, Prasad N R et al. (2013) Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxidative medicine and cellular longevity. 102741.
- 8.Moon Y, Lee K H, Park J H, Geum D, Kim K. (2005) Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. , Journal of neurochemistry 93, 1199-208.
- 9.Miller R L, James-Kracke M, Sun G Y, Sun A Y. (2009) Oxidative and inflammatory pathways in Parkinson's disease. , Neurochemical research 34, 55-65.
- 10.Terzi A, Iraz M, Sahin S, Ilhan A, Idiz N et al. (2004) Protective effects of erdosteine on rotenone-induced oxidant injury in liver tissue. , Toxicology and industrial health 20, 141-7.
- 11.Hall A M, Campanella M, Loesch A, Duchen M R, Unwin R J. (2010) Albumin uptake in OK cells exposed to rotenone: a model for studying the effects of mitochondrial dysfunction on endocytosis in the proximal tubule?. , Nephron Physiology 115, 9-19.
- 12.Sureka B, Bansal K, Patidar Y, Rajesh S, Mukund A et al. (2015) Neurologic Manifestations of Chronic Liver Disease and Liver Cirrhosis. Current problems in diagnostic radiology. 44, 449-61.
- 13.Arnold R, Issar T, Krishnan A V, Pussell B A. (2016) . Neurological complications in chronic kidney disease. JRSM cardiovascular disease 5: 2048004016677687.
- 14.Chillon J M, Massy Z A, Stengel B. (2016) Neurological complications in chronic kidney disease patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association -. , European Renal Association 31, 1606-14.
- 15.Balzano T, Forteza J, Molina P, Giner J, Monzo A et al. (2018) . The Cerebellum of Patients with Steatohepatitis Shows Lymphocyte Infiltration, Microglial Activation and Loss of Purkinje and Granular Neurons. Scientific reports 8: 3004.
- 16.Bronas U G, Puzantian H, Hannan M. (2017) Cognitive Impairment in Chronic Kidney Disease: Vascular Milieu and the Potential Therapeutic Role of Exercise. BioMed research international 2726369.
- 17.Hasan W, Kori R K, Thakre K, Yadav R S, Jat D. Tehran University of Medical Sciences (2019) Synthesis, characterization and efficacy of mitochondrial targeted delivery of TPP-curcumin in rotenone-induced toxicity. Daru : journal of Faculty of Pharmacy.
- 18.Panche A N, Diwan A D, Chandra S R. (2016) Flavonoids: an overview. , Journal of nutritional science 5-47.
- 19.Salehi B, Machin L, Monzote L, Sharifi-Rad J, Ezzat S M et al. (2020) Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. , Mutha RE, Tatiya AU, Surana SJ, ACS 5, 11849-72.
- 21.Zhao Y, Chen B, Shen J, Wan L, Zhu Y et al. (2017) . The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid Med Cell Longev 1459497.
- 22.Robak J, Gryglewski R J. (1988) Flavonoids are scavengers of superoxide anions. , Biochemical pharmacology 37, 837-41.
- 23.Chan H M, Zhu L F, Zhong R, Grant D, Goyer R A et al. (1993) Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicology and applied pharmacology 123:. 89-96.
- 24.Mohamed Isa SSP, Ablat A, Mohamad J. (2018) The Antioxidant and Xanthine Oxidase Inhibitory Activity of Plumeria rubra Flowers. , Molecules 23.
- 25.Tauber A I, Fay J R, Marletta M A. (1984) Flavonoid inhibition of the human neutrophil NADPH-oxidase. , Biochemical pharmacology 33, 1367-9.
- 26.Gordon M H, Roedig-Penman A. (1998) Antioxidant activity of quercetin and myricetin in liposomes. Chemistry and physics of lipids 97:. 79-85.
- 27.Ishige K, Schubert D, Sagara Y. (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free radical biology. , medicine 30, 433-46.
- 28.Geetha T, Malhotra V, Chopra K, Kaur I P. (2005) Antimutagenic and antioxidant/prooxidant activity of quercetin. , Indian journal of experimental biology 43, 61-7.
- 29.Ohkawa H, Ohishi N, Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. , Anal Biochem 95, 351-8.
- 30.Hasan M, Haider S S. (1989) Acetyl-homocysteine thiolactone protects against some neurotoxic effects of thallium. , Neurotoxicology 10, 257-61.
- 31.Miranda K M, Espey M G, Wink D A. (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric oxide : biology and chemistry 5:. 62-71.
- 32.Kakkar P, Das B, Viswanathan P N. (1984) A modified spectrophotometric assay of superoxide dismutase. , Indian journal of biochemistry & biophysics 21, 130-2.
- 34.Lowry O H, Rosebrough N J, Farr A L, Randall R J. (1951) Protein measurement with the Folin phenol reagent. , The Journal of biological chemistry 193, 265-75.
- 35.Boyde A, Reid S A. (1983) A new method of scanning electron microscopy for imaging biological tissues. , Nature 302, 522-3.
- 36.Rai D K, Sharma B. (2007) Carbofuran-induced oxidative stress in mammalian brain. , Molecular biotechnology 37, 66-71.
- 37.Sharma P, Singh R. (2012) Dichlorvos and lindane induced oxidative stress in rat brain: Protective effects of ginger. , Pharmacognosy research 4, 27-32.
- 38.Abdou H M, Hussien H M, Yousef M I. (2012) Deleterious effects of cypermethrin on rat liver and kidney: protective role of sesame oil. , Journal of environmental science. Part B, Pesticides, food contaminants, and agricultural wastes. 306-14.
- 39.Xu D, Hu M-J, Wang Y-Q, Cui Y-L. (2019) Antioxidant activities of quercetin and its complexes for medicinal application. , Molecules 24, 1123.
- 40.Pfeiffer R F. (2003) Gastrointestinal dysfunction in Parkinson's disease. , Lancet Neurol 2, 107-16.
- 41.Edwards L L, Quigley E M, Pfeiffer R F. (1992) Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. , Neurology 42, 726-32.
- 42.Sharma N, Jamwal S, Kumar P. (2016) Beneficial effect of antidepressants against rotenone induced Parkinsonism like symptoms in rats. , Pathophysiology 23, 123-34.
- 43.Najafabadi R E, Kazemipour N, Esmaeili A, Beheshti S, Nazifi S. (2018) Quercetin Prevents Body Weight Loss Due to the Using of Superparamagnetic Iron Oxide Nanoparticles in Rat. Advanced biomedical research. 7, 8.
- 44.Contreras-Zentella M L, Hernandez-Munoz R. (2016) Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxidative medicine and cellular longevity. 3529149.
- 45.Giannini E G, Testa R, Savarino V. (2005) Liver enzyme alteration: a guide for clinicians. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 172, 367-79.
- 46.Akinmoladun A C, Oladejo C O, Josiah S S, Famusiwa C D, Ojo O B et al. (2018) Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology : the official journal of the International Society for Pathophysiology. 25, 365-71.
- 47.Liu X, Zhang Y, Liu L, Pan Y, Hu Y et al. (2020) Protective and therapeutic effects of nanoliposomal quercetin on acute liver injury in rats. , BMC Pharmacology and Toxicology 21, 1-7.
- 48.Kim S Y, Moon A. (2012) Drug-induced nephrotoxicity and its biomarkers. , Biomol Ther (Seoul) 20, 268.
- 49.Kataria A, Trasande L, Trachtman H. (2015) The effects of environmental chemicals on renal function. , Nature reviews Nephrology 11, 610-25.
- 50.Weber E J, Himmelfarb J, Kelly E J. (2017) Concise Review: Current and Emerging Biomarkers of Nephrotoxicity. Current opinion in toxicology. 4, 16-21.
- 51.Cao X, Nie X, Xiong S, Cao L, Wu Z et al. (2018) Renal protective effect of polysulfide in cisplatin-induced nephrotoxicity. Redox biology. 15, 513-21.
- 52.Feriani A, M del, Talhaoui N, Gómez-Caravaca A M, Taamalli A et al. (2017) Protective effect of Globularia alypum leaves against deltamethrin-induced nephrotoxicity in rats and determination of its bioactive compounds using high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole–time-of-flight mass spectrometry. Journal of functional foods. 32, 139-48.
- 53.Sindhu G, Nishanthi E, Sharmila R. (2015) Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemical and molecular study. , Environ Toxicol Pharmacol 39, 392-404.
- 54.Amin K A, Ahmed R R, Hozayen W G, Antar A. (2017) Renoprotective and antioxidant effects of silymarin and propolis on diclofenac sodium-induced renal toxicity in rats. , International Journal for Pure and Applied Bioscience 5, 31-42.
- 55.Hassan S S, Thomann C, Ettarh R, Ahmad Z. (2017) Possible protective role of silybin against polymyxin E-induced toxic effect in rat kidneys: A biochemical approach. , Neurourol Urodyn 36, 2003-10.
- 56.Jiang X W, Qiao L, Feng Liu L, Wei Q W, Wang X W. (2017) Rotenone induces nephrotoxicity in rats: oxidative damage and apoptosis. , Toxicol Mech Methods 27, 528-36.
- 57.Soliman K M, Abdul-Hamid M, Othman A I. (2007) Effect of carnosine on gentamicin-induced nephrotoxicity. , Med Sci Monit 13, 73-83.
- 58.Crown O, Ogundele O, Akinmoladun A, Famusiwa C, Josiah S et al. (2019) Effects of catechin, quercetin and taxifolin on redox parameters and metabolites linked with renal health in rotenone-toxified rats. , Niger J Physiol Sci 34, 1-10.
- 59.Li S, Cao C, Shi H, Yang S, Qi L et al. (2016) Effect of quercetin against mixture of four organophosphate pesticides induced nephrotoxicity in rats. , Xenobiotica 46, 225-33.
- 60.Hussein H, Elnaggar M, Al-Dailamy J. (2012) Protective role of Vitamin C against hepatorenal toxicity of fenvalerate in male rats. , Global Advanced Research Journal of Environmental Science and Toxicology 1, 060-5.
- 61.Halliwell B. (2007) Biochemistry of oxidative stress. , Biochemical Society transactions 35, 1147-50.
- 62.Uzun F G, Kalender Y. (2013) Chlorpyrifos induced hepatotoxic and hematologic changes in rats: the role of quercetin and catechin. Food Chem Toxicol. 55, 549-56.
- 63.Renugadevi J, Prabu S M. (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 62, 471-81.
- 64.Gupta S S, Azmi L, Mohapatra P K, Rao C V. (2017) Flavonoids from whole Plant of Euphorbia hirta and their Evaluation against Experimentally induced Gastroesophageal Reflux Disease in Rats. Pharmacognosy magazine. 13, 127-34.
- 65.Dogan Z, Elbe H, Taslidere E, Soysal H, Cetin A et al. (2017) Effects of ciprofloxacin on fetal rat liver during pregnancy and protective effects of quercetin. Biotechnic & histochemistry : official publication of the Biological Stain Commission 92, 481-6.
- 66.Amirtharaj G J, Natarajan S K, Pulimood A, Balasubramanian K A, Venkatraman A et al. (2017) Role of Oxygen Free Radicals, Nitric Oxide and Mitochondria in Mediating Cardiac Alterations During Liver Cirrhosis Induced by ThioacetamidZ Cardiovascular toxicology. 17, 175-84.
- 67.Manna E, Bank S, Maiti S, Jana P, Sinha A K. (2015) Neutralization by Acetyl Salicylic Acid of the Testosterone induced Impaired Maspin Synthesis Stimulated by Estriol in Neutrophils through Nitric Oxide Synthesis. International journal of biomedical science : IJBS. 11, 176-84.
- 68.Liang H L, Whelan H T, Eells J T, Wong-Riley M T. (2008) Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. , Neuroscience 153, 963-74.
- 69.Jiang X, Feng X, Huang H, Liu L, Qiao L et al. (2017) The effects of rotenone-induced toxicity via the NF-kappaB-iNOS pathway in rat liver. Toxicology mechanisms and methods. 27, 318-25.
- 70.Hasan W, Kori R K, Jain J, Yadav R S, Jat D. (2019) Neuroprotective effects of mitochondria-targeted curcumin against rotenone-induced oxidative damage in cerebellum of mice. Journal of biochemical and molecular toxicology. 22416.
- 71.Ojha A, Srivastava N. (2012) Redox imbalance in rat tissues exposed with organophosphate pesticides and therapeutic potential of antioxidant vitamins. Ecotoxicol Environ Saf. 75, 230-41.
- 72.Lasram M M, Lamine A J, Dhouib I B, Bouzid K, Annabi A et al.Antioxidant and anti-inflammatory effects of N-acetylcysteine against malathion-induced liver damages and immunotoxicity in rats. , Life Sci 107, 50-8.
- 73.Boadi W Y, Amartey P K, Lo A. (2016) Effect of quercetin, genistein and kaempferol on glutathione and glutathione-redox cycle enzymes in 3T3-L1 preadipocytes. Drug and chemical toxicology. 39, 239-47.
- 74.Xu D, Hu M J, Wang Y Q, Cui Y L. (2019) Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. , Molecules 24.
- 75.Kocahan S, Dogan Z, Erdemli E, Taskin E. (2017) Protective Effect of Quercetin Against Oxidative Stress-induced Toxicity Associated With Doxorubicin and Cyclophosphamide in Rat Kidney and Liver Tissue. Iranian journal of kidney diseases. 11, 124-31.
- 76.Porras D, Nistal E, Martinez-Florez S, Pisonero-Vaquero S, Olcoz J L et al. (2017) Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free radical biology &. medicine102: 188-202.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
International Journal of Life Science and Pharma Research (2023) OpenAlex
International Journal of Pharmaceutical Sciences and Research (2023) OpenAlex