Reaction Norm of Embryo Growth Rate Dependent on Incubation Temperature in The Olive Ridley Sea Turtle, Lepidochelys Olivacea, from Pacific Central America
Abstract
Olive ridleys (Lepidochelys olivacea) and loggerheads (Caretta caretta) are two closely phylogenetically related sea turtles that nest in very different thermal habitat. Olive ridleys nest in pan-tropical beaches whereas loggerheads nest in more temperate beaches. In the context of climate change, the temperature in temperate beaches will increase much more than for tropical beach due to buffering effect of air humidity in the later. We have determined the thermal reaction norm for embryonic growth in both species using field records of incubation temperatures and incubation length from loggerheads in Western Mediterranean Sea or olive ridleys from Pacific coast of Guatemala. We show that the optimum temperature for the growth of embryos is lower for loggerheads than for olive ridleys. This makes loggerhead turtles particularly sensitive to increase of beach temperature as it is expected due to effect of global warming in temperature regions. Furthermore, olive ridleys are more resilient to increase of temperatures and should not suffer from sublethal incubation temperatures.
Author Contributions
Academic Editor: Adriana Cortés-Gómes, Laboratoire d'Ecologie, Systématique et Evolution. Université Paris-Sud, Email: [email protected]
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Berta Alejandra Morales-Mérida, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Global warming presents major challenges to organisms 1, 2. There is a pressing need in conservation biology and climate change research for cross-species, quantitative, objective criteria for assessing the susceptibility of species to climate change-induced extinction 3. Overall, current estimates of biodiversity loss due to climate change are very variable, depending on the method, taxonomic group, metrics, spatial and seasonal scales considered 4. Yet, the majority of studies indicate alarming consequences for biodiversity, with the worst-case scenarios leading to extinction rates that would qualify as the sixth mass extinction in the history of the earth 5.
One attempt to address this shortcoming is phenomenological, using climatic descriptions of contemporary ranges coupled with expected temperatures to predict future ranges 6. These bioclimatic models are best case scenarios because, while they reflect existing interspecific differences in projected ranges under global warming, they also implicitly assume that all species have similar potential to access and exploit predicted climate space. The insidious threat from climate change is that it will weaken or preclude ecological responses by species that are physiologically constrained; yet, quantitative, objective criteria for assessing relative susceptibility of diverse taxa to warming-induced stress are wanting 3. Consequently, biologists are endeavouring to develop robust ways to evaluate the differential vulnerability of organisms to climate change 7 and then to evaluate and implement management strategies tailored for species judged most at risk 8.
The extent of adaptive variation in heat-tolerance is likely to have important consequences for the resilience of many ectothermic species in a rapidly warming world 9, 10, 11, 12, 13. This has led to calls for an ‘adaptive evolutionary’ approach to conservation, which seeks to conserve functional diversity (rather than simply genetic marker diversity) at whatever scale it occurs 14.
Lepidochelys olivacea, the olive ridley, and Caretta caretta, the loggerhead, are two phylogenetically closely related sea turtles 15 that nest in very different thermal habitats. Olive ridley turtles nest in intertropical zone while loggerheads nest in more temperate beaches 16. Virtually all biological rates are affected by temperature including development duration 17 and growth rates 18 of ectothermic animals. Egg incubation temperatures affect the duration of embryogenesis 19, the probability of embryo survival 20, 21, the sex determination for species with temperature-dependent sex determination such as Caretta caretta22 and the performance, the morphology, and body size at hatching 21, 23. In addition, long-term effects of incubation temperature on the physiology and behaviour of hatchlings has been observed 24, 25. Thus, the fitness of developing embryos is strongly dependent on the temperature within the nest during incubation. Figure 1
Figure 1.Map of Central America with greyed Guatemala. Monterrico nesting beach is indicated by a black point within the Monterrico Natural Reserve for Multiple Uses (Reserva Natural de Usos Múltiple Monterrico).
Recently, a general way to model sigmoidal embryo growth with variable incubation temperature during development obtained from in situ sea turtle nests has been proposed and tested with data from Caretta caretta from Dalyan beach, Turkey 26. New data of incubation temperatures and incubation durations have been gathered in 2011-2012 for Lepidochelys olivacea nesting in Guatemala Pacific coast. These data have permitted to estimate the thermal reaction norm for the embryo growth for this species and to compare this pattern with the loggerhead one.
Materials and Methods
Field Data
Olive Ridley sea turtles nest along all of the Pacific Coast of Guatemala. Egg collection for human consumption is authorized as long as 20% of the eggs of each collected nest is given to hatcheries located along all the littoral 27. A total of 1,600 eggs collected on the previous night were bought to various collectors. The dates of incubation beginning were as followed: 16/11/2011 - 140 eggs; 22/11/2011 - 120 eggs; 23/11/2011 - 160 eggs; 24/11/2011 - 1000 eggs; 25/11/2011 - 160 eggs; 26/11/2011 - 20 eggs. We had no control on how the eggs were handled during the previous night but the hatching success was very high. Eggs were grouped in 80 nests (20 randomly selected eggs per nest) in this experiment. Among them, 40 have been incubated in hatchery (hatchery nests) and 40 have been used in 4 different experiments (experimental nests). Half of these 40 nests were buried in sand at 40 cm depth and half at 60 cm depth. Among each group of these 40 experimental nests, half (10) have been incubated in open beach under full shade and half (10) in full sun. Experiment was conducted at the hatchery of the Monterrico Natural Reserve for Multiple Uses.
Analysis of Incubation Data
Incubation time, longitudinal ttemperatures and 10 hatchling straight carapace lengths at the nearest 0.1 mm were registered for all monitored nests. Mean incubation temperatures and incubation durations were analysed using linear model 28 and hatchling sizes were analysed using linear mixed model with nest identity as a random factor 29. In all cases, fixed factors were depth of nests (40 or 60 cm) and with or without shade and their interaction. Mean incubation temperature and its first order interaction with other factors were added for analysis of mean incubation duration and hatchling size. A backward model selection was used by removing the least non-significant factor one at a time. A single factor was not removed if it was significant when involved in an interaction. F test after ANOVA (ANalysis Of VAriance) was used to detect the influence of factors after linear model 28 and Likelihood Ratio Test (LRT) test after ANOVA was used to detect the influence of factors after linear mixed model using R package glmmADMB 30.
The variability of daily temperatures among nests was measured as the mean of the daily standard deviation of temperatures recorded in nests from each treatment (shading status and depth). Welch modified two-sample t-test with unequal variances were used to test the treatments effect (depth and shading) 31.
Thermal Growth Rate Reaction Norm
The model of embryo growth integrates in a single framework both the growth rate dependency on temperature and the embryo growth 26. The parameters for growth rate dependency on temperature that maximized the logarithm of the likelihood (Ln L) of the observed hatchling size distribution were search for using the R package embryogrowth 32. The model is summarized here briefly but a complete description can be found in the original publication 26.
Biological temperature-dependent rate models based on Arrhenius’ and Eyring’s equations have been formulated by Sharpe and DeMichele 33. The original formulation of Sharpe and DeMichele was modified by Schoolfield et al. 34 to remove the very high correlations of parameter estimators. Two kinds of equations using 4 or 6 parameters produced a curve with a maximum at an intermediate temperature and decreased bellow and above this temperature. The level as well as the position of the maximum can be manipulated using the parameters values.
The early growth of embryos is modelled using a modification of the Gompertz model 35 proposed by Laird 36 (eqn 1):
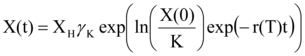 eqn 1
eqn 1
Where X(0) is the size or mass at nesting time (time=0), r(T) is the growth rate at the beginning of the curve, and K is the carrying capacity with
XH is the hatchling size and rK=2.09 is a constant used to slowdown growth at the end of incubation 26 to ensure that embryological stages are well positioned during incubation.
The dynamic of X (t )is governed by the Gompertz differential equation (eqn 2):
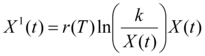 eqn 2
eqn 2
The gastrula is approximately a disk of 1.7 mm diameter and this size will be used as X (0) 37.
Model Fitting
Growth rate r(T) can be calculated with models (4 or 6 parameters) from Schoolfield et al. 34 model and an incubation temperature T. With X(0) and K, and a time-series of r(T), the pattern of change of embryo size for this nest is evaluated using Runge-Kutta method of order 4 for the approximation of solutions of ordinary differential equations.
Estimation of parameters was performed using maximum likelihood with an identity link and a Gaussian distribution of SCL. The standard error of parameters was estimated using the square-root of the inverse of the Hessian matrix which is an asymptotic approximation of the variance-covariance matrix 38. The models are implemented in the R package embryogrowth 32.
Comparison Between Sets of Data
First, growth rate r(T) has been fitted for hatchery and experimental Lepidochelys olivacea nests separately. Next, all the nests were grouped in a single dataset and growth rate r(T) has been fitted again. We used AIC and Akaike weight to select between 4 and 6-parameters models. AIC is a measure of the relative quality of fit, which penalized for too many parameters in the model 39 and Akaike weight gives the relative statistical support of several models tested on the same dataset 40. Likelihood ratio test has been used to test whether a single model for hatchery and experimental nests was sufficient or not to describe observed data.
The statistics 
(Likelihood Ratio Test, with Ln L being the logarithm of the likelihood) is distributed as a χ2 with the degrees of freedom being the difference of number of parameters between the most complete model and the simplest one 41.
Results
Analysis of Incubation Data
Distribution of temperatures recorded in the 80 Lepidochelys olivacea nests from the beach of Monterrico, Guatemala are shown in Figure 2 as well as the temperatures recorded in Caretta caretta nests from Turkey 42. Average incubation temperatures for the 80 nests ranges from 29.11 °C to 33.56 °C (mean=31.00 °C, sd=1.53 °C). Shaded nests were significantly cooler than those exposed to the sun by 2.11 °C (paired t-tests with Bonferonni correction, p<10-9). Nests from hatchery were also significant cooler than the experimental ones (paired t-tests with Bonferonni correction, 1.32 °C difference between hatchery vs shaded, p<10-9 and 3.44 °C difference hatchery vs sun, p<10-9) (Figure 3A). Among the experimental nests, only shading status was significant  to explain the difference between nests for average incubation temperatures. Depth and interaction between shading status and depth were not significant
to explain the difference between nests for average incubation temperatures. Depth and interaction between shading status and depth were not significant and
and 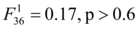 respectively).
respectively).
Figure 2.Temperatures recorded in hatchery (A) and experimental (B) Lepidochelys olivacea nests from Monterrico, Guatemala. As a comparison, temperatures recorded in 21 Caretta caretta nests from Turkey are shown 42 (C).
Incubation duration ranged from 43 to 55 days (mean=49.86 days, sd=3.63). Significant effect of mean incubation temperature and shading status for experimental data was observed  and
and  respectively) but not of depth as well as all interactions (all p>0.1). Incubation duration was longer for cooler temperatures and shaded nests (Figure 3B).
respectively) but not of depth as well as all interactions (all p>0.1). Incubation duration was longer for cooler temperatures and shaded nests (Figure 3B).
Figure 3.Descriptive statistics of incubation data for Lepidochelys olivacea nests from Monterrico, Guatemala plotted according to the significant factors explaining the differences between treatments. (A) Mean incubation temperature, (B) incubation duration, and (C) hatchling size.
Straight carapace length of hatchlings (mean 40.86 mm, sd=1.82) was significantly different for experimental data according to depth factor (deviance= 5.492, df=1, p<0.02) but not for any other factors (all p>0.05). Embryos incubated at 40 cm were smaller (40.22 mm, sd=1.54 mm) than those incubated at 60 cm (41.07 mm, sd=1.36 mm) (Figure 3C).
An effect of shading at 40 cm (t = -6.7727, df = 9.15,p < 0.0001) and 60 cm (t = 5.0015, df = 11.441, p< 0.001) and depth for shade (t = -2.3516, df = 15.382, p-value < 0.04) and sun-exposed nests (t = 2.1895, df = 11.557, p < 0.05) were noticed on the daily standard deviation temperatures.
Temperature Dependent Embryonic Growth Rate
Parameters maximizing likelihood of observed hatchling size for each nest have been fitted first using the total set of 80 nests using the 4 and the 6-parameters equation describing instantaneous growth rate dependency to temperature. AIC for 4-parameters model was 380.97 whereas it was 390.05 for the 6-parameters model. Akaike weight gives a very strong support to retain the 4-parameters model (p=0.99). In a second step, parameters have been fitted separately for hatchery nests (Ln L=-97.83), for experimental nests (Ln L=-79.79) and for all nests together (Ln L=-186.55) with LRT being 1.90 (df=4, p=0.75). Thus, a single model for the two categories of nests was sufficient.
Figure 4.Average of the daily standard deviation of temperatures recorded in nests from each treatment (shading status and depth), for Lepidochelys olivacea nests from Monterrico, Guatemala.
Figure 5.Fitted straight carapace length (SCL) depending on incubation time for the 80 monitored nests of Lepidochelys olivacea, from Monterrico, Guatemala. The horizontal lines are the observed hatching size and twice the standard deviation.
The fitted pattern of embryo growth for the 80 nests is shown in Figure 5. It should be noted that all fitted embryo sizes at the end of the incubation are comprised within the 95% confidence interval of observed hatchling sizes.
The fitted instantaneous growth rate according to temperature is shown in Figure 6 for Lepidochelys olivacea from Guatemala. The curve fitted for Mediterranean Caretta caretta42, 43 is also shown for comparison.
Figure 6.Fitted growth rate r(T) of straight carapace length (SCL) depending on incubation temperature T for Lepidochelys olivacea (this study) and Caretta caretta 43. The envelope in dashed lines is the 2.5% and 97.5% quantiles.
Discussion
Temperature during incubation of ectothermic animals can have profound consequences on the fitness of individuals and then selection should act to adapt response of embryos to temperature.
The effect of shading on incubation temperature was anticipated based on several previous reports for turtles 44, 45, 46, 47 but also lizards 48. Indeed, we found that the shaded experimental nests have a lower mean incubation temperature than the experimental nests exposed to the sun. Hatchery nests are also shaded and have still a lower temperature (Figure 3A). However, we did not detect a significant effect of depth probably because the differential between both depths (40 and 60 cm) was not sufficient to produce enough change in temperature. As a consequence, shaded nests take longer to emerge when compared to the nests directly exposed to sun and incubation temperature could modulate this effect (Figure 3B).
Hatchlings from eggs incubated at 40 cm were significantly smaller than those from eggs incubated at 60 cm (Figure 3C) but a direct effect of temperature or shading was not observed. We do not have definitive explanation for this effect. Mass of hatchling has been shown to be dependent on incubation temperature in many reptiles 49, 50, 51, 52 but also on dryness of substrate 53. On the other hand, effect on size is not consistent among studies nor within the same study. For example, no effect has been observed in the turtle Pelodiscus sinensis23 but a slight decrease of carapace length has been measured in the sea turtle Caretta caretta as the moisture increased 54. But, in the same experiment and for the same turtles, carapace width and plastron length did not show any consistent pattern of increasing with higher moisture 54.
Here we do not detect an effect of temperature on size but an effect of depth of the nest. This effect could be mediated by moisture difference between two depths: it is possible that eggs incubated at 40 cm lost more water that those located at 60 cm, the latter being closer to the water table (sea water that infiltrates by porosity in sand) 55. Eggs incubated in dryer substrate accumulate less water during incubation 56. The mechanism linking substrate water content and size of embryos has been well studied in the freshwater Chrysemys picta 57. Water within the eggs plays a key role in the mobilization of yolk reserves by reptile embryos and consequently influences hatchling mass 57.
Caretta caretta nests mainly out of the intertropical region whereas Lepidochelys olivacea has a distribution more centred on the equator 16. The northernmost nest for a sea turtle has been deposited by a Caretta caretta in South of France at the latitude 43°16.05'N 58. As a consequence, the nest temperatures experienced by Lepidochelysolivacea (Figure 2A and B) are generally much higher than temperatures experienced by Caretta caretta (Figure 2C). The growth rate depending on temperature in olive ridleys showed a pattern of increase from 20 °C to 35 °C. However, it should be noted that the part of the curve from 20 °C to 27 °C is extrapolated based on Schoolfield model of thermal reaction norm 34 because no lower temperature than 27 °C was recorded in Lepidochelys olivacea nests from Guatemala (Figure 2). On the other hand, temperature as low as 22 °C was recorded in Caretta caretta nests in Turkey 42 and the pattern of growth rate for these two species at low temperatures was very similar (Figure 6). However, the pattern differs completely for incubation at high temperatures. The growth rate continued to increase up to 35 °C for Lepidochelys olivacea whereas it went down for Caretta caretta around 32 °C. This is consistent with the observation that incubation duration increased at 32 °C as compared to 31°C 43 and that no Caretta caretta hatchlings survived when incubated at 32 °C and above 21.
The variability of temperatures among different nests incubated in same conditions was measured. We detect an effect of depth but much more an effect of shading (Figure 4). Such a measure is particularly important because it permits to explain why, in the same beach and at the same time, heterogeneity of incubation conditions was observed. The thermal microhabitats have been overlooked for sea turtle and generally for reptiles. This is particularly important in the context of prediction of the impact of climate change. The climate models available give prediction at the regional scale 59 very far away from the beach scale and even intra-nesting beach level as shown here. This downscaling is challenging to be able to produce realistic models of incubation of reptile eggs.
Acknowledgments
We thank the hatchery of the Monterrico Natural Reserve for Multiple Uses and the authorities of Guatemala for authorization to use the eggs collected for this experiment. We thank two anonymous referees for their very valuable suggestions that have enhanced the clarity of this manuscript and we thank also the editor Adriana Cortés-Gómes for her help with this manuscript.
References
- 1.Parmesan C. (2006) Ecological and evolutionary responses to recent climate change. , Annu. Rev. Ecol. Syst 37, 637-669.
- 2. (2007) Intergovernmental Panel on Climate Change.Climate change 2007: the physical science basis.CambridgeUniversityPress:Cambridge,UK.
- 3.Bernardo J, Ossola R J, Spotila J, Crandall K A. (2007) Interspecies physiological variation as a tool for cross-species assessments of global warming-induced endangerment: validation of an intrinsic determinant of macroecological and phylogeographic structure. , Biol. Lett 3, 695-699.
- 4.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. (2012) Impacts of climate change on the future of biodiversity. , Ecol. Lett 15, 365-377.
- 5.Pereira H M, Leadley P W, Proença V, Alkemade R, Scharlemann J P W. (2010) Scenarios for global biodiversity in the 21st century. , Science 330(6010), 1496-1501.
- 6.Bellard C, Leclerc C, Courchamp F. (2014) Impact of sea level rise on the 10 insular biodiversity hotspots. , Glob. Ecol. Biogeogr 23(2), 203-212.
- 7.Williams S E, Shoo L P, Isaac J L, Hoffmann A A, Langham G. (2008) Towards an integrated framework for assessing the vulnerability of species to climate change. , PLoS Biol 6, 2621-2626.
- 8.Lee T M, Jetz W. (2008) Future battlegrounds for conservation under global change. , Proc. R. Soc. B 275, 1261-1270.
- 9.Baker A C, Starger C J, McClanahan T R, Glynn P W. (2004) Coral reefs: corals’ adaptive response to climate change. , Nature 430, 741.
- 10.Jensen L F, Hansen M M, Pertoldi C, Holdensgaard G, Mensberg KLD. (2008) Local adaptation in brown trout early life-history traits: implications for climate change adaptability. , Proc R Soc B 275, 2859-2868.
- 11.Baskett M L, Gaines S D, Nisbet R M. (2009) Symbiont diversity may help coral reefs survive moderate climate change. , Ecol. Appl 19, 3-17.
- 12.Somero G N. (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. , J. Exp. Biol 213, 912-920.
- 13.Eliason E J, Clark T D, Hague M J, Hanson L M, Gallagher Z S. (2011) Differences in thermal tolerance among sockeye salmon populations. , Science 332(6025), 109-112.
- 14.Fraser D J, Bernatchez L. (2001) Adaptive evolution ary conservation: towards a unified concept for defining conservation units. , Mol. Ecol 10, 2741-2752.
- 15.Guillon J-M, Guéry L, Hulin V, Girondot M. (2012) A large phylogeny of turtles (Testudines) using molecular data. , Contrib. Zool 81(3), 147-158.
- 16.B P Wallace, A D DiMatteo, B J Hurley, E M Finkbeiner, A B Bolten. (2010) Regional management units for marine turtles: a novel framework for prioritizing conservation and research across multiple scales. PLoS. , One 5(12), 15465.
- 17.J F Gillooly, E L Charnov, G B West, V M Savage, J H Brown. (2002) Effects of size and temperature on developmental time. , Nature 417, 70-73.
- 18.J F Gillooly, J H Brown, G B West, V M Savage, E L Charnov. (2001) Effects of size and temperature on metabolic rate. , Science 293, 2248-2251.
- 19.J D Miller. (1985) Embryology of marine turtles. , In: C Gans, F Billet, PF Maderson (eds). Biology of the Reptilia. Wiley-Liss: New-York, US 270-328.
- 20.R Van Damme, Bauwens D, Brana F, R F Verheyen. (1992) Incubation temperature differentially affects hatching time, egg survival, and hatchling performance in the lizard Podarcis muralis. , Herpetologica 48(2), 220-228.
- 21.L R Fisher, M H Godfrey, D W Owens. (2014) Incubation temperature effects on hatchling performance in the loggerhead sea turtle (Caretta caretta). , PLoS One 9(12), 114880.
- 22.Pieau C. (1996) Temperature variation and sex determination in reptiles. , Bioessays 18(1), 19-26.
- 23.Du W-G, Ji X. (2003) The effects of incubation thermal environments on size, locomotor performance and early growth of hatchling soft-shelled turtles, Pelodiscus sinensis. , J. Therm. Biol 28, 279-286.
- 24.R M Sibly, Atkinson D. (1994) How rearing temperature affects optimal adult size in ectotherms. , Funct. Ecol 8, 486-493.
- 25.Burger J. (1989) Incubation temperatures has long-term effects on behaviour of yound pine snakes (Pituophis melanoleucus). , Behavioural Ecology and Sociobiology 24, 201-207.
- 26.Girondot M, Kaska Y. (2014) A model to predict the thermal reaction norm for the embryo growth rate from field data. , J. Therm. Biol 45, 96-102.
- 27.Juarez R, Muccio C. (1997) Sea turtle conservation in Guatemala. , Marine Turtle Newsletter 77, 15-17.
- 28.McCullagh P, J A Nelder. (1989) Generalized Linear Models, Second edition edn.Chapman and Hall:. , New York, USA
- 29.Stroup W W. (2012) Generalized Linear Mixed Models: Modern Concepts, Methods and Applications.Chapman and Hall:Boca. , Raton, Florida
- 30.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. (2016) . Generalized Linear Mixed Models using ‘AD Model Builder’.0.8.3.3Revision: 284.
- 31.B L Welch. (1947) The generalization of “student’s”problem when several different population variances are involved. , Biometrika 34, 28-35.
- 32.Girondot M. (2017) embryogrowth: Tools to analyze the thermal reaction norm of embryo growth. 7.0 ed: The Comprehensive R Archive Network.
- 33.Sharpe PJH, DeMichelle D W. (1977) Reaction kinetics of poikilotherm development. , J. Theor. Biol 64, 649-670.
- 34.Schoolfield R M, Sharpe P J, Magnuson C E. (1981) Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. , J. Theor. Biol 88(4), 719-731.
- 37.Kaska Y, Downie R. (1999) Embryological development of sea turtles (Chelonia mydas. , Caretta caretta) in the Mediterranean. Zool. Middle East 19, 55-69.
- 38.Abt M, Welch W J. (1998) Fisher information and maximum-likelihood estimation of covariance parameters in Gaussian stochastic processes. , Can. J. Stat.-Rev. Can. Stat 26(1), 127-139.
- 39.Akaike H. (1974) A new look at the statistical model identification. , IEEE Trans. Autom. Control 19, 716-723.
- 40.K P Burnham, D R Anderson. (2002) Model selection and multimodel inference: A practical information-theoretic approach.Springer-Verlag:. , New York
- 42.Girondot M, Kaska Y. (2014) Nest temperatures in a loggerhead-nesting beach in Turkey is more determined by sea surface temperature than air temperature. , J. Therm. Biol 47, 13-18.
- 43.Monsinjon J, Jribi I, Hamza A, Ouerghi A, Kaska Y. (2017) Embryonic growth rate thermal reaction norm of Mediterranean Caretta caretta embryos from two different thermal habitats, Turkey and Libya. Chelonian Conserv. Biol., In press.
- 44.F J Janzen. (1994) Vegetational cover predicts the sex ratio of hatchling turtles in natural nests. , Ecology 75(6), 1593-1599.
- 45.J L Schmid, D S Addison, M A Donnelly, M A Shirley, Wibbels T. (2008) The effect of Australian Pine (Casuarina equisetifolia) removal on Loggerhead Sea Turtle (Caretta caretta) incubation temperatures on Keewaydin Island. , Florida. J. Coast. Res.,55(sp1) 214-220.
- 46.J M Refsnider, D A Warner, F J Janzen. (2013) Does shade cover availability limit nest-site choice in two populations of a turtle with temperature-dependent sex determination?. , J. Therm. Biol 38(3), 152-158.
- 47.J E Hill, F V Paladino, J R Spotila, P S Tomillo. (2015) Shading and watering as a tool to mitigate the impacts of climate change in sea turtle nests. , PLoS One 10(6), 0129528.
- 48.J S Doody, Guarino E, Georges A, Corey B, Murray G. (2006) Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. , Evol. Ecol 20, 307-330.
- 49.Brana F, Ji X. (2000) Influence of incubation temperature on morphology, locomotor performance, and early growth of hatchling wall lizards (Podarcis muralis). , J. Exp. Zool 286(4), 422-433.
- 50.Ji X, W G Du. (2001) The effects of thermal and hydric environments on hatching success, embryonic use of energy and hatchling traits in a colubrid snake, Elaphe carinata. Comparative Biochemistry and Physiology A-Molecular and Integrative Physiology,129(2-3):. 461-471.
- 51.Ji X, W G Du. (2001) Effects of thermal and hydric environments on incubating eggs and hatchling traits in the cobra, Naja naja atra. , J. Herpetol 35(2), 186-194.
- 52.Ji X, Qiu Q B, Diong C H. (2002) Influence of incubation temperature on hatching success, energy expenditure for embryonic development, and size and morphology of hatchlings in the oriental garden lizard, Calotes versicolor (Agamidae). , J. Exp. Zool 292(7), 649-659.
- 53.Delmas V, Bonnet X, Girondot M, Prévot-Julliard A-C. (2008) Varying hydric conditions during incubation influence egg water exchange and hatchling phenotype in the red-eared slider turtle. , Physiol. Biochem. Zool 81(3), 345-355.
- 54.McGehee M A. (1990) Effects of moisture on eggs and hatchlings of loggerhead sea turtles (Caretta caretta). , Herpetologica 46(3), 251-258.
- 55.Nielsen P. (1990) Tidal dynamics of the water table in beaches. , Water Resour. Res 26(9), 2127-2134.
- 56.M S Finkler. (1999) Influence of water availability during incubation on hatchling size, body composition, desiccation tolerance, and terrestrial locomotor performance in the snapping turtle Chelydra serpentina. , Physiol. Biochem. Zool 72, 714-722.
- 57.G C Packard, M J Packard. (2001) Environmentally induced variation in size, energy reserves and hydration of hatchling painted turtles, Chrysemys picta. , Funct. Ecol 15, 481-489.
- 58.Sénégas J-B, Hochscheid S, Groul J-M, Lagarrigue B, Bentivegna F. (2008) Discovery of the northernmost loggerhead sea turtle (Caretta caretta) nest. , JMBA Biodiversity Records 6269, 1-4.
- 59.Christensen J H, Hewitson B, Busuioc A, Chen A, Gao X. (2007) Regional climate projections. In:S.Solomon, D.Qin, M.Manning, Z.Chen, M.Marquis, KB.Averyt, et al.(eds).Climate Change2007:The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.Cambridge University Press:Cambridge,United Kingdom and New York,NY,USA.

