Preparation of Microwave Multi-Adsorbent Nanocomposites Based on Copper, Iron Carbonyl, Carbon Nanofiber, Graphite Nanoflake and Polypyrrole
Abstract
The composites of Cu, Carbonyl iron (CI), carbon nanofiber (CNF), graphite nanoflake (GNF)/polypyrrole (PPy) and [(Cu-CI-CNF-GNF) 0.5-PAA]-PPy0.5 were synthesized via different methods by in-situ polymerization on the surface of nanoparticles (NPs) with core-shell structure. This paper describes a method for polyacrylic acid (PAA) coating of NPs in aqueous solution. Then PPy coating was performed by template polymerization on NPs-PAA. Morphology, magnetic and conductivity properties were observed via scanning electron microscopy (SEM), vibrating sample magnetometer (VSM) and four probe method, respectively. The microwave characterization of nanocomposite was evaluated through arch test based on a network analyzer. The PPy nanocomposites possessed the excellent microwave multi absorbers properties in 2-18 GHz. It was also found that nanocomposites with 50% w/w and light weight exhibit good microwave absorbing properties in 2-3 GHz and 5-14 GHz frequency, so can be used to cellphone, radio frequency and radar shielding.
Author Contributions
Academic Editor: Fazal Raziq, School of Physics, University of Electronic Science and Technology of China.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Seyed Hossien Hosseini
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In the last decade, various microwave (MW) absorption materials have been widely investigated for electromagnetic interference to protect human health and electronic equipment from electromagnetic pollution which is caused by the wide applications of high-power electronic devices and communication technology 1, 2. High absorption, wide frequency band and low density are a pursuing in the design of microwave absorption materials. Some current researches of electromagnetic absorption are focused on the range from 2-18 GHz 3, 4. Polypyrrole (PPy) and polyaniline (PANI), the most extensively studied conducting polymers, have attracted great interest in the construction of electromagnetic absorbers for its low weight, high electrical conductivity and suitable Physical chemistry properties 5, 6. For instance, MWCNT/Ba0.2Sr0.2La0.6MnO3 nanocomposite based PANI was synthesized by core-shell structure which exhibit Ku band absorption property 7. Increased absorption of electromagnetic waves based on nanoparticles and carbon nanostructures has also received much attention 8. The multiband microwave absorption films epoxy-based multilayered coating containing carbon nanotube (CNT), silicon carbide (SiC), and carbonyl iron (CI) particles were prepared by Mahdavi and his coworkers 9. In addition to absorbing electromagnetic waves, polypyrrole and epoxy nanocomposites also increase enhanced flame retardancy in the presence of nanomagnetite 10. On the other hand, nanocomposites with shell core structure have been extensively developed and have several applications 11, 12. The conductive and magnetic nanocomposites with core-shell and different nanostructures were used for electromagnetic absorption application 13, 14. The preceding works, we have synthesized magnetic and conductive nanofiber-nanocomposites based polythiophene which exhibit excellent MW absorption in the X-band range 15. Therefore, we fabricated double core-shell structure polyaniline nanocomposites which exhibit both good MW absorption and thermal infrared performance 16, 17, 18. The graphite nanoflake (GNF) and carbon nanofiber (CNF) as a new kind of absorbers have attracted much attention for their unique physical, chemical and mechanical properties, such as their light weight, flexibility, high specific surface and excellent electronic conductivity 15, 19. In this work, we attempted to add Cu and CI nanoparticles into the CNF, GNF and PPy composite to synthesize a new nanocomposite with five components, [(Cu-CI-GNF)0.5-PPA]-PPy0.5.A green chemical method was applied to prepare by in-situ polymerization on the surface of all components after were coated by PAA. This paper is a revised and expanded microwave absorption properties in the frequency of 2-18 GHz.
Experimental
Materials
Natural flake graphite with an average size of 500 µ m was used for preparing the expanded graphite nanoflakes. Concentrated sulfuric acid and concentrated nitric acid were used as chemical intercalate and oxidizers. Pyrrole monomer (analytical grade, Merck) distilled twice under reduced pressure and stored blew 00C. The liquid carbonyl iron was commercially from Aldrich. Carbon nanofiber was purchased size of 10 to 20 nm industrial. Dodecylbenene sulfuric acid (DBSA, 90%) and polyacrlic acid (PAA) were purchased from the Aldrich. All the other chemical reagents were purchased from Merck without further purification.
Preparation of Green Cu Nanoparticles
For biological synthesis of copper nanoparticles, Nag champa (Artabotrys odoratissimus, Family: Annonaceae), leaves were collected and dried for 4 days at room temperature. The plant leaf broth solution was prepared by taking 25 g of thoroughly washed and finely cut leaves in a 1 L beaker with 500 mL of sterile distilled water and then boiling the mixture for 5 min before finally decanting it. It was stored at 4 0C and used within a week. Typically, 30 mL of leaf broth was added to 170 mL of 1 mmolL−1 aqueous CuSO4.5H2O solution for the reduction of copper ions. The effects of temperature on synthesis rate and particle size of the prepared copper nanoparticles were studied by carrying out the reaction in a water bath at 95 oC with reflux. The copper nanoparticle solution thus obtained was purified by repeated centrifugation at 15,000 rpm for 20 min followed by re-dispersion of the pellet in deionized water.
Preparation of Graphite Nanoflake (GNF)
A mixture of concentrated sulfuric acid and nitric acid (3:1, v/v) was mixed with graphite flake at room temperature. The reaction mixture was stirred continuously for 12 h. The acid treated natural graphite was washed with water until neutralized and was then dried at 60 oC to remove any remaining water. The dried flakes were heat-treated at 1050 oC for 15s to obtain expanded graphite. Expanded graphite was immersed in a 70% of aqueous alcohol solution in an ultrasonic bath. The mixture was sonicated for 12 h, and then was filtered and dried to produce GNF.
Coating of NPs with PAA (NPs-PAA)
0.5 g NPs and 50 mL PAA (5% w/v) were added into 250 mL flask and the mixture were ultrasonicated for 15 min. The mixture was stirred vigorously at 25 oC for 24 h. The mixture was filtered and then washed with acetic acid (2% v/v) and acetone. After vacuum drying the filtrate, NPs-PAA was achieved.
Preparation of PPy Nanocomposite, [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5
The PPy nanocomposite as core-shell nanocomposite was prepared with template polymerization by in-situ polymerization in the presence of DBSA as the surfactant and dopant and Fe(NO3)3.9H2O as the oxidant. The 0.5 g DBSA dissolved in distilled water with vigorous stirring for about 20 min. The 0.287 g NPs-PAA were added to the DBSA solution under sitirring condition for approximately 1 h .Then 1 mL (0.015 mol) of freshly distilled pyrrole as monomer added to the suspension and stirred for 30 min. The NPs-PAA were dispersed well in the mixture of PPy/DBSA under ultrasonication for 2 h. 12.12 g (0.03 mol) Fe(NO3)3.9H2O as intiator dissolved in 30 mL deionized water and added drop wise to stirred reaction mixture. Polymerization was allowed to proceed for 6 h. The nanocomposite was obtained by filtering was washing the suspension with deionized water and aceton, respectively. The obtained dark powder contains [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5 and dried under vacuum for 24 h.
Characterization
The ultrasonic experiment was carried out by an ultrasonic disperser (Hielsche, UP4005, Germany). Field emission scanning electron microscopy (FESEM) was performed by TESCAN MIRA to observe surface morphologies of samples. The magnetic measurements carried out at room temperature using a Termo company 7400 model (USA), vibrating sample magnetometer (VSM) with maximum magnetic of 10 KOe. The XRD patterns of the samples were collected on a Philips-PW 1800 with Cu Kα radiation (λ=1.54184 Å) in the 2θ= 4-900 with steps of 0.020, scanning operated at 40 kV and 30 mA (Netherland). The electrical conductivity of compressed pellet of samples and nonocomposites were calculated using a standard four-probe set-up connected to a Keithly system comprising a voltmeter and constant high-current source, made in IRAN. Microwave absorption properties of nanocomposites were measured using microwave vector network analyzer (Agilent technologies Inc.8722-USA) in the 2-18 GHz range at room temperature.
Results and Discussion
FTIR Study
(Figure 1) shows FTIR spectrum of [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5 nanocomposites. We synthesized GNF by acid treatment and thermal shock. We expect see hydroxyl and carboxyl functional group on GNF. So, the band at 3439 cm-1 can be attributed to O-H stretching (st.) vibrations of alcoholic functional group presented on the GNF, PAA and DBSA. And attributed to N-H st. vibrations of PPy, The peak at 2922 and 2845 cm-1 are attributed to aliphatic C-H st. vibrations of PAA and DBSA. The peaks at 2359, 2332 cm-1 and 1675, 1643 cm-1 are related to C=O st. vibration of CI, PAA and DBSA, respectively. The different angles of Fe-C=O have been obtained for CI, so we observed to higher frequency for C=O. The specific peaks around 1537 and 1461 cm-1 are attributed with vibrational modes of quinonic and aromatic type ring for PPy. The peaks at 1385,1461 cm-1 are attributed to C=C st. vibration of CNF and GNF that 1461 cm-1 cover to aromatic mode with PPy. The peaks at 1171 and 1046 cm-1 are attributed to C-N and C-C st. vibration mode for PPy and CNF, GNF, respectively. The peaks at 779, 665 and 601, 517 cm-1 are attributed to Cu-O and Fe-O st. vibration for Cu and CI NPs.
XRD Patterns Study
(Figure 2) shows XRD pattern for GNF that the peak at 2θ=26 related to carbon of GNF. The XRD patterns of molecular structures of CI-PAA-PPy, Cu-PAA-PPy and CNF-PAA-PPy in Figure 3 (a-c), were showed, respectively. The results show the diffraction peaks of NPs structures were not destroyed after the chemical polymerization of PPy as shell. We see all patterns of core NPs and shell polymers well. According to Figure 3 (a-c) characteristic peaks at 2θ=16-25, 2θ=17-25, 42, 44, 48, 50,72.38 and 2θ=16-23 with base peaks at 2θ=22.33, 72.38 and 24.83 for the CI-PAA-PPy, Cu-PAA-PPy and CNF-PAA-PPy were observed that correspond to JCPDS files no. 4073-004-98, 1646-002-98 and 2719-008-98, respectively. Figure 2 shows XRD pattern for GNF that the peak at 2θ=26 related to GNF. The XRD patterns showed molecular structures of CI-PAA-PPy, Cu-PAA-PPy and CNF-PAA-PPy in Figure 3 (a-c), respectively. The results show the diffraction peaks of NPs structures were not destroyed after the chemical polymerization of PPy as shell. We see all patterns of core NPs and shell polymers well, too.
Figure 2.XRD pattern for GNF that the peak at 2θ
Figure 3.XRD of a) CI-PAA-PPy , b) Cu-PAA-PPy and c) CNF-PAA-PPy
According to Figure 3 (a-c) characteristic peaks at 2θ=16-25, 2θ=17-25, 42, 44, 48, 50,72.38 and 2θ=16-23 with base peaks at 2θ=22.33, 72.38 and 24.83 were observed for the CI-PAA-PPy, Cu-PAA-PPy and CNF-PAA-PPy that correspond to JCPDS files no. 4073-004-98, 1646-002-98 and 2719-008-98, respectively. These results and the relative width of the peaks indicate that the nanocomposite containing NPs-PAA-PPy are semi-crystalline and amorphous. According to these results and peaks observed in XRD patterns, we can ensure the existence of NPs. The average crystallite size can be calculated by the Debye-scherrer formula: D= 0.89 λ/β cosθ where λ is the wavelength of Cu Kα radiation and the value of K depends on serveral factors, including the Miller index of reflection plane and the shape of the crystal.
If the shape is unknown, K is often assigned as a value of 0.89, D is average crystallite size, θ is the Bragg’s angle, and β is the full width at half-maximum of the diffraction peaks. Therefore, from the width of the peaks observed in the XRD patterns, the average crystallite sizes of GNF, CI, Cu and CNF are calculated to 70, 21.5, 20.3 and 19.8 nm, respectively.
SEM Images Study
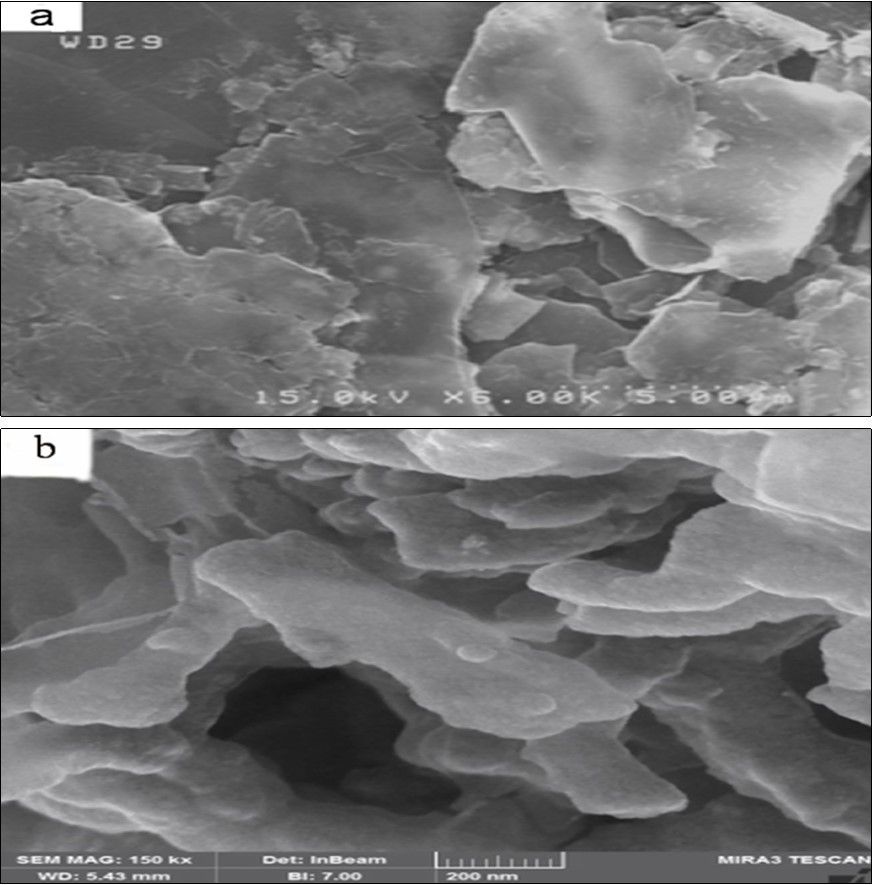
(Figure 4) (a-d) shows FESEM images of Cu, CI NPs and Cu-PAA-PPy, CI-PAA-PPy nanocomposites. The diameters of samples are about 30, 59, 40 and 65 nm, respectively. Figure 5 (a-b) shows the FESEM image of the CNF and CNF-PAA-PPy. Images analysis calculation results showed that the average diameters of CNF and its nanocomposite are 18 and 55 nm, respectively. Figure 6 (a,b) shows the FESEM images of the GNF and GNF-PAA-PPy nanocomposite. We adopted ultrasonic irradiation technique to break down the expanded graphite and then obtained GNF. The average distribution of all NPs, both pure and in the composite substrate, is very good and well dispersed. This will have a direct effect on their absorption properties. Image analysis calculation results showed that the average sheet diameter is approximately 5µ m, and average thickness of nanoflake is about 80 nm. In Figure 6 b, we can see that PPy was coated on the most of GNF. All NPs are completely coated by PPy. The thickness of PPy as shell in all nanocomposites are about 10-20 nm. The surfaces of SEM images of nanocomposites were shown uniformity with some hollow and sponge structures. This structure, which is porous and has composite cavities, helps to reduce the energy of microwaves. By observing the shapes of nanofibers and nanoflakes and their composites, it is clear that the nanofibers in the composite are completely blurred, while the nanosheets have almost retained their shape in the composite.
Figure 4.FESEM images of a) Cu b) CI NPs and c) Cu-PAA-PPy d) CI-PAA-PPy nano composite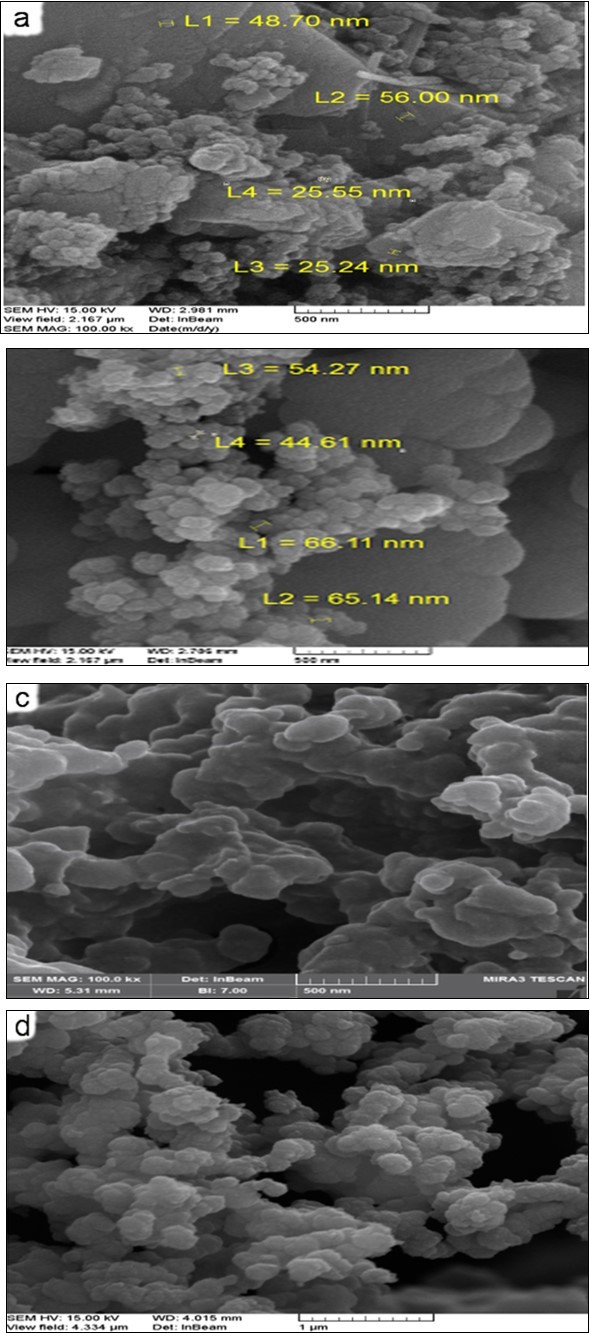
Figure 5.FESEM image of the a) CNF and b) CNF-PAA-PPy
Vibrating Sample Magnetometer (VSM) Study
The magnetization curves versus the magnetic field of CI NPs and CI-PAA-PPy nanocomposite are shown in Figure 7 (a,b). The samples are ferromagnetic behavior and applied fields are 20 and 30 kOefor CI NPs and CI-PAA-PPy respectively. The magnetic parameters such as saturation magnetization (Ms), coercivity (Hc) and remnant magnetization (Mr) are measured by the hysteretic loops. As shown in Figure 7 (a,b), the value of Ms decreased from 225.87 to 51.164 emu/g and the value of Mr increased from 1.882 to15.452 emu/g for CI to nanocomposite. On the other hands, Hc was increased from 16.558 to 151.53 Oe, too. VSM curve of nanocomposite showed semi hard magnetization behavior that can be used to magnetic memories and microwave absorber.
Figure 7.Vibrating sample magnetometer (VSM) field of a) CI NPs and b) CI-PAA-PPy nanocomposite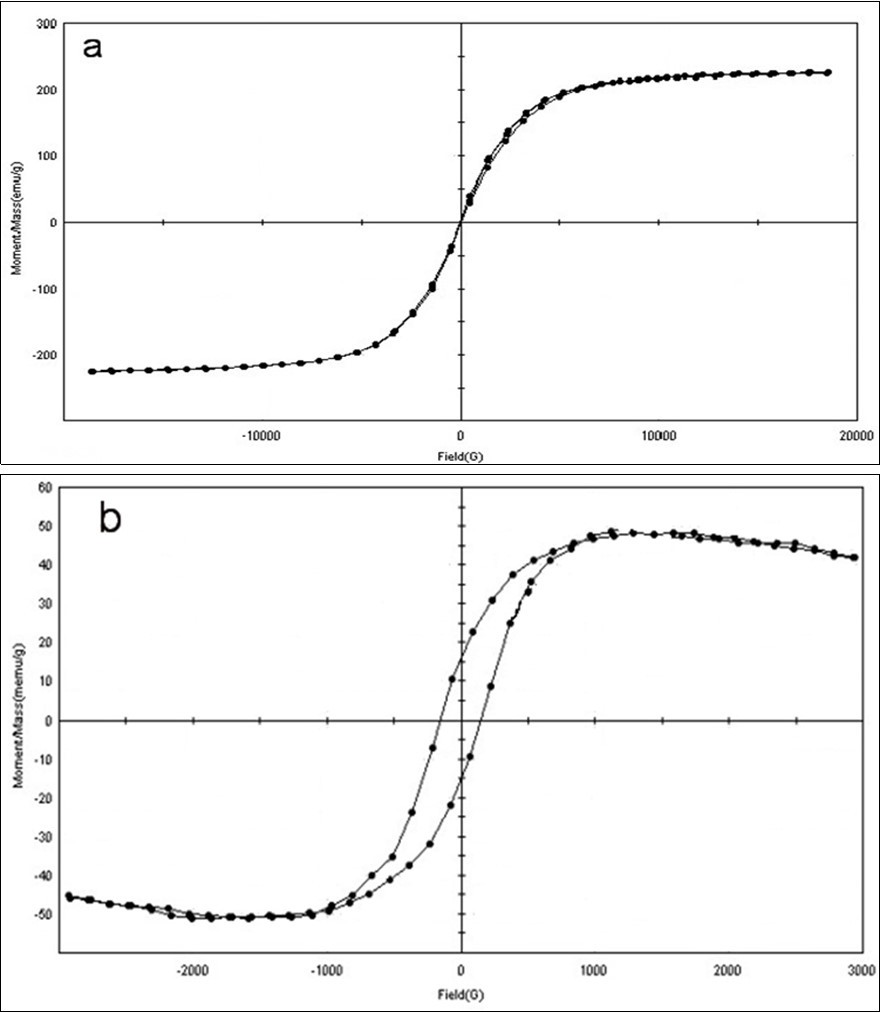
Electrical Conductivity Study
Electrically conductivity of NPs and their nanocomposites were measured by four probe method and were summarized in Table 1. The conductivity of PPy after polymerization by Fe(III) as initiator and DBSA as dopant is 0.044 S/cm. The conductivity of Cu that prepared by chemically method is higher than green method. This is due to completely of reduction in chemical method. On the other hands, conductivity of CNF is higher than GNF that is related to high molecular weight and crystallity of GNF. When mass content of Cu, CNF and GNF as core and PPy as shell were incorporated in composites, conductivities are increased. But incorporation of CI NPs, conductivity is decreased.
Table 1. The electrical conductivity of samples| Sample | Conductivity (S/cm) | Sample | Conductivity (S/cm) |
| Cu (chemical) | 245 | Cu-PAA-PPy | 133 |
| Cu (green) | 170 | CI-PAA-PPy | 0.083 |
| GNF | 20 | GNF-PAA-PPy | 0.88 |
| CNF | 264 | CNF-PAA-PPy | 320 |
| PPy (doped) | 0.044 | [(Cu-CI-CNF-GNF)0.5-PAA] -PPy0.5 | 280 |
| PPy (undoped) | 1.4×10-6 |
Microwave Absorbing Study
The microwave absorbing properties of nanocomposites with the coating thickness of 1 mm investingated by using vector network analyzers in the frequency range of 2-18 GHz, that this range is contained S, H, C, X and Ku bands.
The results for PPy, Cu-PAA-PPy, CI-PAA-PPy and [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5 nanocomposite are shown in Figure 8. The results for PPy, CNF-PAA-PPy , GNF-PAA-PPy, and [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5 are shown in Figure 9. The best microwave absorption were obtained in 9.5 GHz for PPy, 3 and 9 GHz for Cu-PAA-PPy, 9.5 GHz for CI-PAA-PPy, 8.5 and 11 GHz for CNF-PAA-PPy, 9.14 and 16.5 GHz for GNF-PAA-PPy with minimum reflection loss in -17.5, -10, -17, -19, -31, -27, -18, -27.5 and -25 dB, respectively. The absorption bandwidth under -10 dB are 3.2, 3.5, 8.4, 12.9, 13 and 15.5 GHz ranging from 2 to 18 GHz for PPy, Cu-PAA-PPy, CI-PAA-PPy, CNF-PAA-PPy, GNF-PAA-PPy and [(Cu-CI-CNF-GNF)0.5-PAA]-PPy0.5 .
Figure 8.Microwave absorbing results for PPy, Cu-PAA-PPy, CI-PAA-PPy, and (Cu -PPy0.5 nanocomposite
Figure 9.Microwave absorbing results for PPy, CNF-PAA-PPy , GNF-PAA-PPy, and (Cu-PPy0.5
Conclusion
Two factors affect the absorption properties of electromagnetic waves: first, the conduction properties and second, the magnetic properties. In the selected samples, iron carbonyl has completely magnetic properties and other nanoparticles have electrical conductivity properties. In addition, the final polymer also has full electrical conductivity. Therefore, in the above project, we tried to put these compounds together with special engineering in order to achieve the maximum absorption in different areas of the microwave. So, we have synthesized green copper powder, GNF and purchased CI, CNF and describe a method for PAA coating on these. Then PPy coating was performed on template polymerization via in-situ method. Finally, we prepared their nanocomposites both separately and complex with core-shell structure. In continue, their microwave absorption properties in range of 2-18 GHz (S, H, C, X and Ku bands) were investigated. The results showed that the optimum absorption are 2-4 GHz and 5-14 GHz with RL of -12.5 and -33 dB and thickness of 1 mm, respectively. The microwave absorption of samples was increased by increasing core and PPy weight ratio and electrical conductivity. These samples can be used to microwave absorption as most multiband absorber for civil and military applications.
Compliance with Ethical Standards
Conflict of interest the authors declare that they have no conflict of interest.
Shah, N., Zaman, T., Rehan, T., Khan, S., Khan, W., Khan, A., Ul-Islam, M. (2019), Preparation and Characterization of Agar Based Magnetic Nanocomposite for Potential Biomedical Applications, Current Pharmaceutical Design 25(34), 3672-3680.
Meazzini, I., Comby, S., Richards, K. D., Withers, A. M., Turquet, F. X., Houston, J. E., Owens, R. M., and Evans, R. C. (2020),Synthesis and characterisation of biocompatible organic–inorganic core–shell nanocomposite particles based on ureasils, J. Mater. Chem. B, 8, 4908-4916.
Binling, C., Yazdani, B., Benedetti, L., Hong, C., Yanqiu, Z., Oana, G., (2019), Fabrication of nanocomposite powders with a core-shell structure, Composites Science and Technology170, 116-127.
References
- 1.Shukla V. (2019) Review of electromagnetic interference shielding materials fabricated by iron ingredients.Nanoscale. , Adv 1, 1640-1671.
- 2.Dawei J, Murugadoss V, Ying.W.,Jing, L.,Tao, D.,Zicheng, W.,Qian, S.,Chao, W.,Hu, L.,Na, L.,Renbo, W.,Subramania, A., Zhanhu(2019), Electromagnetic interference shielding polymers and nanocomposites-a review.Polym. , Rev 59, 280-337.
- 3.Yongli D, Zhihua X, Xiaoya Y, Zhenfei G, Yushu T et al. (2018) . , Enhanced Electromagnetic Microwave Absorption Property of Peapod-like MnO@carbon Nanowires,ACS Appl. Mater. Interfaces 10, 40078-40087.
- 4.Li J, Lu W, Suhr J, Chen H, J Q Xiao et al. (2017) Superb electromagnetic wave-absorbing composites based on large-scale graphene and carbon nanotube films.Synthesis and. Processing7 2349-2354.
- 5.Chang X, Fan W, Aming X, Liqun D, Zhiqian Y et al. (2020) Hollow Polypyrrole Nanofiber-Based Self-Assembled Aerogel: Large-Scale Fabrication and Outstanding Performance in Electromagnetic Pollution Management. , Ind. Eng.Chem. Res 59, 7604-7610.
- 6.S H Hosseini, Tarakameh M, S. (2020) Preparation of thermal neutron absorber based B4C/TiO2/polyaniline nanocomposite,Int. , J. Phys. Sci.15 49-59.
- 7.S H Hosseini, Paymanfar R, Afshari T, S. (2020) Preparation of MWCNT/Ba0.2Sr0.2La0.6MnO3/PANI nanocomposites and investigation of its electromagnetic properties in KU-band,Int. , J. Phys. Sci.15 131-141.
- 8.Biao Z, Yang L, Xiaoqin G, Rui Z, Jiaoxia Z et al. (2019) Enhanced electromagnetic wave absorbing nickel (oxide)-carbon nanocomposites,Ceram. 45(18), 24469-24473.
- 9.Ghanbari F, Moradi S, Mahdavi H. (2020) Epoxy-based multilayered coating containing carbon nanotube (CNT), silicon carbide (SiC), and carbonyl iron (CI) particles: as efficient microwave absorbing materials,Journal of. , Coatings Technology and Research 17, 815-826.
- 10.Young K, P D, Xinyu L, Xin Z, Jie K et al. (2017) Polypyrrole-interface-functionalized nano-magnetite epoxy nanocomposites as electromagnetic wave absorber with enhanced flame retardancy,J. , Mater. Chem 5, 5334-5344.
- 11.Shah N, Zaman T, Rehan T, Khan S, Khan W et al. (2019) Preparation and Characterization of Agar Based Magnetic Nanocomposite for Potential Biomedical Applications,Current Pharmaceutical Design25(34). 3672-3680.
- 12.Meazzini I, Comby S, K D Richards, A M Withers, F X Turquet et al. (2020) and characterisation of biocompatible organic–inorganic core–shell nanocomposite particles based on ureasils,J. , Mater. Chem. B 8, 4908-4916.
- 13.S H Hosseini, Zamani P, S Y Mousavi. (2015) Thermal infrared and microwave absorbing ofSrTiO3/SrFe12O19/polyaniline nanocomposites.J. , Alloy Comp 644, 423-429.
- 14.Hui L, Guangzhen.C.,Ling, L.,Zhi, Z.,Xuliang, L.,Xinxin, W.(2020), Polypyrrole chains decorated on CoS spheres: A core-shell like heterostructure for high-performance microwave absorption,Nanomaterials 10. 166.
- 15.S H Hosseini, Alamian A, S M Mousavi. (2014) Perpration of magntice and conductive graphite nano flakes/SrFe12O19/polythiophene nanofiber-nanocomposite and its radar absorbing application.Fibers and polymers17. 593-599.
- 16.S H Hosseini, Zamani P. (2016) Preparation of thermal infrared and microwave absorber using. SrTiO3/BaFe12O19/polyaniline nanocomposites.Journal of Magnetism and Materials397 205-211.
- 17.M B Dolabi, Azimi A, S H Hosseini. (2020) Preparation of thermal infrared and microwave absorber using WO3/MnFe3O4/polyaniline nanocomposites.Materials Research. Innovations24 326-334.
Cited by (6)
This article has been cited by 6 scholarly works according to:
Citing Articles:
Nanomanufacturing (2023) Crossref
S. H. Hosseini, Amir Abbas Kazemi, Seyed Arash Hosseini - Nanomanufacturing (2023) Semantic Scholar
Nanomanufacturing (2023) OpenAlex
SSRN Electronic Journal (2022) Crossref
SSRN Electronic Journal (2022) OpenAlex
Journal of Electronic Materials (2022) Crossref
S. H. Hosseini, M. B. Dolabi - Journal of Electronic Materials (2022) Semantic Scholar
Journal of Electronic Materials (2022) OpenAlex